Abstract
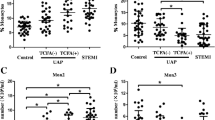
Inflammation plays a pivotal role in coronary heart disease. Dendritic cells (DCs) are principal players in inflammation and atherosclerosis. Although the percentage of circulating DC precursors in coronary heart disease have been investigated, circulating myeloid DC (mDC) and plasmacytoid DC (pDC) precursors have not been extensively studied, particularly in relation to the severity of coronary artery lesions in patients with coronary heart disease. In this study, we recruited controls (n = 29), patients with stable angina pectoris (SAP, n = 30), patients with unstable angina pectoris (UAP, n = 56), and patients with acute myocardial infarction (AMI, n = 50). The severity and extent of coronary artery lesions was evaluated by Gensini score, following coronary angiograms. The percentage of circulating mDC and pDC precursors was determined by fluorescence-activated cell sorting (FACS). Plasma levels of MCP-1 and MMP-9, which correlate with atherosclerosis and DC migration, were also measured. The percentage of circulating mDC precursors was reduced in patients with AMI and UAP compared with control and SAP patients, respectively (p < 0.01 for AMI vs. SAP and Control, p < 0.05 for UAP vs. SAP and Control). The percentage of circulating pDC precursors was not significant changed. The levels of plasma MMP-9 and MCP-1 and Genisi score were all increased in patients with AMI and UAP, compared to control and SAP patients, respectively (p < 0.01 for AMI vs. SAP and control, p < 0.05 for UAP vs. SAP and control). Overall, the percentage of circulating mDC precursors was negatively correlated with MCP-1 (p < 0.001), MMP-9 (p < 0.001) and Genisi scores (p < 0.001). Genisi scores were positively correlated with the levels of MCP-1 (p < 0.001) and MMP-9 (p < 0.001). Our study suggested that the percentage of circulating mDC precursors is negatively correlated with the severity and extent of coronary artery lesions in patients with coronary heart disease.

Similar content being viewed by others
References
Mirzaei M, Truswell AS, Taylor R, Leeder SR (2009) Coronary heart disease epidemics: not all the same. Heart 95:740–746
Hansson GK (2005) Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 352:1685–1695
Galkina E, Ley K (2009) Immune and inflammatory mechanisms of atherosclerosis (*). Annu Rev Immunol 27:165–197
Bobryshev YV (2010) Dendritic cells and their role in atherogenesis. Lab Invest 90:970–984
Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K (2000) Immunobiology of dendritic cells. Annu Rev Immunol 18:767–811
Robinson SP, Patterson S, English N, Davies D, Knight SC, Reid CD (1999) Human peripheral blood contains two distinct lineages of dendritic cells. Eur J Immunol 29:2769–2778
Niessner A, Weyand CM (2010) Dendritic cells in atherosclerotic disease. Clin Immunol 134:25–32
Bobryshev YV, Lord RS (1995) Ultrastructural recognition of cells with dendritic cell morphology in human aortic intima. Contacting interactions of vascular dendritic cells in athero-resistant and athero-prone areas of the normal aorta. Arch Histol Cytol 58:307–322
Paulson KE, Zhu SN, Chen M, Nurmohamed S, Jongstra-Bilen J, Cybulsky MI (2010) Resident intimal dendritic cells accumulate lipid and contribute to the initiation of atherosclerosis. Circ Res 106:383–390
Jongstra-Bilen J, Haidari M, Zhu SN, Chen M, Guha D, Cybulsky MI (2006) Low-grade chronic inflammation in regions of the normal mouse arterial intima predisposed to atherosclerosis. J Exp Med 203:2073–2083
Liu P, Yu YR, Spencer JA, Johnson AE, Vallanat CT, Fong AM, Patterson C, Patel DD (2008) CX3CR1 deficiency impairs dendritic cell accumulation in arterial intima and reduces atherosclerotic burden. Arterioscler Thromb Vasc Biol 28:243–250
Yilmaz A, Lochno M, Traeg F, Cicha I, Reiss C, Stumpf C, Raaz D, Anger T, Amann K, Probst T, Ludwig J, Daniel WG, Garlichs CD (2004) Emergence of dendritic cells in rupture-prone regions of vulnerable carotid plaques. Atherosclerosis 176:101–110
Bobryshev YV, Lord RS (2005) Co-accumulation of dendritic cells and natural killer T cells within rupture-prone regions in human atherosclerotic plaques. J Histochem Cytochem 53:781–785
Kawahara I, Kitagawa N, Tsutsumi K, Nagata I, Hayashi T, Koji T (2007) The expression of vascular dendritic cells in human atherosclerotic carotid plaques. Hum Pathol 38:1378–1385
Yilmaz A, Weber J, Cicha I, Stumpf C, Klein M, Raithel D, Daniel WG, Garlichs CD (2006) Decrease in circulating myeloid dendritic cell precursors in coronary artery disease. J Am Coll Cardiol 48:70–80
Fu Q, Li ZL, Lei X, Fu XH, Yan QN, Liu YF (2008) Peripheral dendritic cell subpopulation changes in patients with coronary artery disease. Zhonghua Xin Xue Guan Bing Za Zhi 36:209–211
Yilmaz A, Reiss C, Weng A, Cicha I, Stumpf C, Steinkasserer A, Daniel WG, Garlichs CD (2006) Differential effects of statins on relevant functions of human monocyte-derived dendritic cells. J Leukoc Biol 79:529–538
Tu Y, Jia R, Ding G, Chen L (2010) Effect of atorvastatin on dendritic cells of tubulointerstitium in diabetic rats. BMB Rep 43:188–192
Niessner A, Sato K, Chaikof EL, Colmegna I, Goronzy JJ, Weyand CM (2006) Pathogen-sensing plasmacytoid dendritic cells stimulate cytotoxic T-cell function in the atherosclerotic plaque through interferon-alpha. Circulation 114(23):2482–2489
Niessner A, Shin MS, Pryshchep O, Goronzy JJ, Chaikof EL, Weyand CM (2007) Synergistic proinflammatory effects of the antiviral cytokine interferon-alpha and Toll-like receptor 4 ligands in the atherosclerotic plaque. Circulation 116(18):2043–2052
Yilmaz A, Lipfert B, Cicha I, Schubert K, Klein M, Raithel D, Daniel WG, Garlichs CD (2007) Accumulation of immune cells and high expression of chemokines/chemokine receptors in the upstream shoulder of atherosclerotic carotid plaques. Exp Mol Pathol 82:245–255
Xu ZX, Yang YZ, Feng DM, Wang S, Tang YL, He F, Xia Y, Li F (2008) Oxidized high-density lipoprotein promotes maturation and migration of bone marrow derived dendritic cells from C57BL/6J mice. Chin Med Sci J 23:224–229
Dong K, Ge JH, Gu SL, Li S, Zhu WG, Fan FY, Zhu JH (2011) Ox-LDL can enhance the interaction of mice natural killer cells and dendritic cells via the CD48-2B4 pathway. Heart Vessels. doi:10.1007/s00380-010-0102-4
Gu L, Okada Y, Clinton SK, Gerard C, Sukhova GK, Libby P, Rollins BJ (1998) Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low-density lipoprotein receptor-deficient mice. Mol Cell 2:275–281
Inoue S, Egashira K, Ni W, Kitamoto S, Usui M, Otani K, Ishibashi M, Hiasa K, Nishida K, Takeshita A (2002) Anti-monocyte chemoattractant protein-1 gene therapy limits progression and destabilization of established atherosclerosis in apolipoprotein E-knockout mice. Circulation 106:2700–2706
Boring L, Gosling J, Cleary M, Charo IF (1998) Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature 394:894–897
Ni W, Kitamoto S, Ishibashi M, Usui M, Inoue S, Hiasa K, Zhao Q, Nishida K, Takeshita A, Egashira K (2004) Monocyte chemoattractant protein-1 is an essential inflammatory mediator in angiotensin II-induced progression of established atherosclerosis in hypercholesterolemic mice. Arterioscler Thromb Vasc Biol 24:534–539
de Lemos JA, Morrow DA, Sabatine MS, Murphy SA, Gibson CM, Antman EM, McCabe CH, Cannon CP, Braunwald E (2003) Association between plasma levels of monocyte chemoattractant protein-1 and long-term clinical outcomes in patients with acute coronary syndromes. Circulation 107:690–695
de Lemos JA, Morrow DA, Blazing MA, Jarolim P, Wiviott SD, Sabatine MS, Califf RM, Braunwald E (2007) Serial measurement of monocyte chemoattractant protein-1 after acute coronary syndromes: results from the A to Z trial. J Am Coll Cardiol 50:2117–2124
Gonzalez-Quesada C, Frangogiannis NG (2009) Monocyte chemoattractant protein-1/CCL2 as a biomarker in acute coronary syndromes. Curr Atheroscler Rep 11:131–138
Jimenez F, Quinones MP, Martinez HG, Estrada CA, Clark K, Garavito E, Ibarra J, Melby PC, Ahuja SS (2010) CCR2 plays a critical role in dendritic cell maturation: possible role of CCL2 and NF-kappa B. J Immunol 184:5571–5581
Hu Y, Ivashkiv LB (2006) Costimulation of chemokine receptor signaling by matrix metalloproteinase-9 mediates enhanced migration of IFN-alpha dendritic cells. J Immunol 176:6022–6033
Yen JH, Khayrullina T, Ganea D (2008) PGE2-induced metalloproteinase-9 is essential for dendritic cell migration. Blood 111:260–270
Ionita MG, Vink A, Dijke IE, Laman JD, Peeters W, van der Kraak PH, Moll FL, de Vries JP, Pasterkamp G, de Kleijn DP (2009) High levels of myeloid-related protein 14 in human atherosclerotic plaques correlate with the characteristics of rupture-prone lesions. Arterioscler Thromb Vasc Biol 29:1220–1227
Chen HQ, Tan HY, Yang YW, Qiu L, Liu XQ (2010) Effects of ramipril on serum monocyte chemoattractant protein 1, interleukin-18, and interleukin-10 in elderly patients with acute coronary syndrome. Heart Vessels 25(2):77–81
Yokota T, Osanai T, Hanada K, Kushibiki M, Abe N, Oikawa K, Tomita H, Higuma T, Yokoyama J, Hanada H, Okumura K (2010) Effects of telmisartan on markers of ventricular remodeling in patients with acute myocardial infarction: comparison with enalapril. Heart Vessels 25(6):460–468
Fukunaga T, Soejima H, Irie A, Fukushima R, Oe Y, Kawano H, Sumida H, Kaikita K, Sugiyama S, Nishimura Y, Ogawa H (2009) High ratio of myeloid dendritic cells to plasmacytoid dendritic cells in blood of patients with acute coronary syndrome. Circ J 73:1914–1919
Erbel C, Sato K, Meyer FB, Kopecky SL, Frye RL, Goronzy JJ, Weyand CM (2007) Functional profile of activated dendritic cells in unstable atherosclerotic plaque. Basic Res Cardiol 102:123–132
Angeli V, Llodra J, Rong JX, Satoh K, Ishii S, Shimizu T, Fisher EA, Randolph GJ (2004) Dyslipidemia associated with atherosclerotic disease systemically alters dendritic cell mobilization. Immunity 21:561–574
Smith SJ, Zheng ZJ (2010) The impending cardiovascular pandemic in China. Circ Cardiovasc Qual Outcomes 3:226–227
Zaguri R, Verbovetski I, Atallah M, Trahtemberg U, Krispin A, Nahari E, Leitersdorf E, Mevorach D (2007) ‘Danger’ effect of low-density lipoprotein (LDL) and oxidized LDL on human immature dendritic cells. Clin Exp Immunol 149:543–552
Blank SE, Johnson EC, Weeks DK, Wysham CH (2010) Circulating dendritic cell number and intracellular TNF-alpha production in women with type 2 diabetes. Acta Diabetol. doi:10.1007/s00592-010-0190-8
Zeyda M, Kirsch BM, Geyeregger R, Stuhlmeier KM, Zlabinger GJ, Horl WH, Saemann MD, Stulnig TM (2005) Inhibition of human dendritic cell maturation and function by the novel immunosuppressant FK778. Transplantation 80:1105–1111
Lapteva N, Ide K, Nieda M, Ando Y, Hatta-Ohashi Y, Minami M, Dymshits G, Egawa K, Juji T, Tokunaga K (2002) Activation and suppression of renin-angiotensin system in human dendritic cells. Biochem Biophys Res Commun 296:194–200
Zhao P, Li XG, Yang M, Shao Q, Wang D, Liu S, Song H, Song B, Zhang Y, Qu X (2008) Hypoxia suppresses the production of MMP-9 by human monocyte-derived dendritic cells and requires activation of adenosine receptor A2b via cAMP/PKA signaling pathway. Mol Immunol 45:2187–2195
Ardans JA, Economou AP, Martinson JJ, Zhou M, Wahl LM (2002) Oxidized low-density and high-density lipoproteins regulate the production of matrix metalloproteinase-1 and -9 by activated monocytes. J Leukoc Biol 71:1012–1018
Nurkic J, Ljuca F, Nurkic M, Jahic E, Jahic M (2010) Biomarkers of plaque instability in acute coronary syndrome patients. Med Arh 64:103–106
Acknowledgments
This study was support by Grant number 2009B030801206 from 2009 Guangdong technology project.
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wen, J., Wen, Y., Zhiliang, L. et al. A decrease in the percentage of circulating mDC precursors in patients with coronary heart disease: a relation to the severity and extent of coronary artery lesions?. Heart Vessels 28, 135–142 (2013). https://doi.org/10.1007/s00380-011-0218-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-011-0218-1