Hyperhomocysteinaemia, a condition characterized as marked elevation of plasma homocysteine, is closely related to atherosclerosis( Reference Guthikonda and Haynes 1 ). Previous studies have observed that a high level of homocysteine is associated with enhanced inflammatory processes, increased oxidative stress and a series of atherothrombotic events( Reference Ridker, Manson and Buring 2 – Reference Curro, Condello and Caccamo 4 ). Therefore, total homocysteine-lowering therapy seems to be a possible practice to reduce the incidence of these outcomes( Reference Yap, Rushe and Howard 5 ). Folate and vitamin B12 are two vital regulators in the metabolic process of homocysteine. Folic acid supplementation might reduce serum homocysteine concentration( Reference Chiuve, Giovannucci and Hankinson 6 , Reference de Bree, Verschuren and Bjorke-Monsen 7 ). Holmes et al. suggested that increasing folate levels with folic acid supplementation might alleviate the effect of methylenetetrahydrofolate reductase MTHFR 677C→T variant on homocysteine concentration( Reference Holmes, Newcombe and Hubacek 8 ). One prospective cohort study showed that a reduction of 25 % (3 μmol/l) in serum homocysteine lowered the risk of stroke by 24 % and the risk of CHD by 18 % in 7·3 years of follow-up( 9 ). However, low folate intake or low plasma folate concentration is associated with increased stroke risk( Reference He, Merchant and Rimm 10 ). In actual practice, the effect of folic acid supplementation to reduce serum homocysteine might be affected by the folate status of a population.
Some randomized controlled trials (RCT) have been performed to investigate folic acid intervention and the associated cardiovascular and cerebrovascular risks. Although several meta-analyses pooled the results of the RCT, they did not reach consensus( Reference Bazzano, Reynolds and Holder 11 – Reference Huo, Qin and Wang 13 ). Significant variance in study characteristics, such as baseline homocysteine level of the participants and whether folate fortification was implemented, were the major sources of heterogeneity. Considering the proved effect of folate fortification on plasma homocysteine control( Reference Holmes, Newcombe and Hubacek 8 ), the present meta-analysis aimed to study the potential effect of folate fortification on folic acid-based homocysteine-lowering intervention and stroke risk by stratifying previous RCT according to fortification status.
Method
Search strategy
The basic framework of the meta-analysis followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement recommended by the Cochrane Collaboration( Reference Liberati, Altman and Tetzlaff 14 ). Relevant studies published between 1 January 1980 and 1 March 2014 were searched in PubMed, Web of Science, Cochrane Library, EMBASE and ClinicalTrials.gov. Introductions and reference lists of previous meta-analyses and reviews were manually searched to avoid possible missing of qualifying trials. Only studies published in English were retrieved since the screening criteria have strict inclusion requisites for reporting of patient baseline and intervention details. The following search strategy and terms were used to identify possible RCT: (‘folate’ OR ‘folic acid’ (All Fields)) AND (‘homocysteine’ (All Fields)) AND (‘stroke’ OR ‘cardiovascular disease’ OR ‘cerebrovascular attack’ OR ‘brain hemorrhage’ OR ‘intracranial hemorrhage’ OR ‘heart attack’ OR ‘angina’ OR ‘myocardial ischemia’ OR ‘myocardial infarct’ (All Fields)) AND (‘randomized controlled trial’ OR ‘RCT’) (All Fields)). Two authors performed the whole search process and assessed the eligibility of the RCT independently. Discrepant judgements were resolved through group discussion.
Inclusion criteria and data extraction
Screening criteria had the following strict inclusion requisites: (i) randomized controlled trial (RCT); (ii) patients were divided into an active treatment group with folic acid supplementation (with or without vitamins B6 and B12) v. a placebo or very-low-dose control group; (iii) folate fortification status of the region where the trial was conducted had to be reported in the study or in relevant studies; (iv) the number of stroke cases had to be reported separately in the active treatment and control groups; (v) the study involved no less than 200 participants; and (vi) follow-up ≥6 months. Two authors extracted original data independently and a third author cross-checked the data. Inconsistencies and discrepancies were resolved by discussion based on reviewing of the original reports. The key data extracted included year of publication, number of patients involved, patients’ baseline folate level/characteristics at study entry, active treatment, length of follow-up, geographical location of the study and results of efficacy and safety outcomes (homocysteine reduction and stroke occurrence).
Statistical analysis
According to the folate fortification status of the trials, studies involved in the present meta-analysis were stratified into subgroups with folate fortification, without folate fortification and with partial folate fortification, respectively. In these three subgroups, the effect of folic acid intervention on homocysteine reduction and stroke risk were compared. Statistical analysis followed the Cochrane Handbook for Systematic Reviews of Interventions Version 5·1·0 (www.cochrane.org) for meta-analysis. To compare the level of homocysteine lowering in the three subgroups, original data on the baseline homocysteine level and median decrease of homocysteine at the end of follow-up were extracted. The level of decrease in each of the three subgroups was calculated as a percentage. The t test for two samples assuming equal variance was performed to check whether there was a significant difference in homocysteine lowering between any two of the three subgroups. Review Manager 5·2 software (Cochrane Collaboration, Oxford, UK) was used for calculation and comparison of treatment effects. Relative risks (RR) with 95 % confidence intervals were calculated for head-to-head comparisons of the association between folic acid supplementation and risk of stroke. A two-tailed P value ≤0·05 was used to denote statistical significance. The heterogeneity between different trials was assessed by χ 2 tests and the I 2 statistic. I 2 values of 25 %, 50 % and 75 % suggest low, moderate and high degree of heterogeneity, respectively( Reference Higgins, Thompson and Deeks 15 ). P<0·1 in χ 2 tests suggests significant heterogeneity. Primary assessment of heterogeneity was conducted based on a fixed-effects model with Mantel–Haenszel methods. P≥0·1 and I 2≤50 % mean the trials have no significant heterogeneity and a fixed-effects model was used, while P<0·1 and I 2>50 % suggest the trials have significant heterogeneity( Reference Higgins, Thompson and Deeks 15 ). Then, the source of heterogeneity was further analysed. If there was no significant clinical or method heterogeneity, a random-effects model was applied to make estimates. Otherwise, descriptive analysis was performed. Results obtained from both fixed-effects and random-effects models were compared to examine the influence of small-study effects on results due to the possible between-study heterogeneity( Reference Higgins, Thompson and Deeks 15 ). Where necessary, sensitivity analyses were performed to test the stability of identified outcomes.
Results
The systematic search found twenty-two articles for detailed assessment. Among them, seven were excluded since no stroke risk was reported( Reference Hodis, Mack and Dustin 16 – Reference de Jager, Oulhaj and Jacoby 22 ), one was excluded due to small patient pool( Reference Righetti, Serbelloni and Milani 23 ), three were excluded due to no baseline report for folate and homocysteine( Reference de Jager, Oulhaj and Jacoby 22 , Reference Liem, van Boven and Veeger 24 , Reference Bostom, Carpenter and Kusek 25 ), one was excluded due to high dose of folate (1 mg/d) as control( Reference Wrone, Hornberger and Zehnder 26 ) and one was excluded due to duplicate study( Reference Liem, Reynierse-Buitenwerf and Zwinderman 27 ). The final fourteen RCT enrolled 39 420 patients. Among them, seven were included in the without folate fortification subgroup( Reference Mark, Wang and Fraumeni 28 – Reference Armitage, Bowman and Clarke 34 ), four were included in the with folate fortification subgroup( Reference Zoungas, McGrath and Branley 35 – Reference House, Eliasziw and Cattran 38 ) and three were included in the partial folate fortification subgroup( Reference Toole, Malinow and Chambless 39 – 41 ). The screening process of potential articles is summarized in a flowchart (Fig. 1). The key characteristics of the fourteen RCT enrolled are presented in Table 1. The baseline folate levels of the participants and their changes in homocysteine during the whole intervention in the trials are summarized in Table 2.
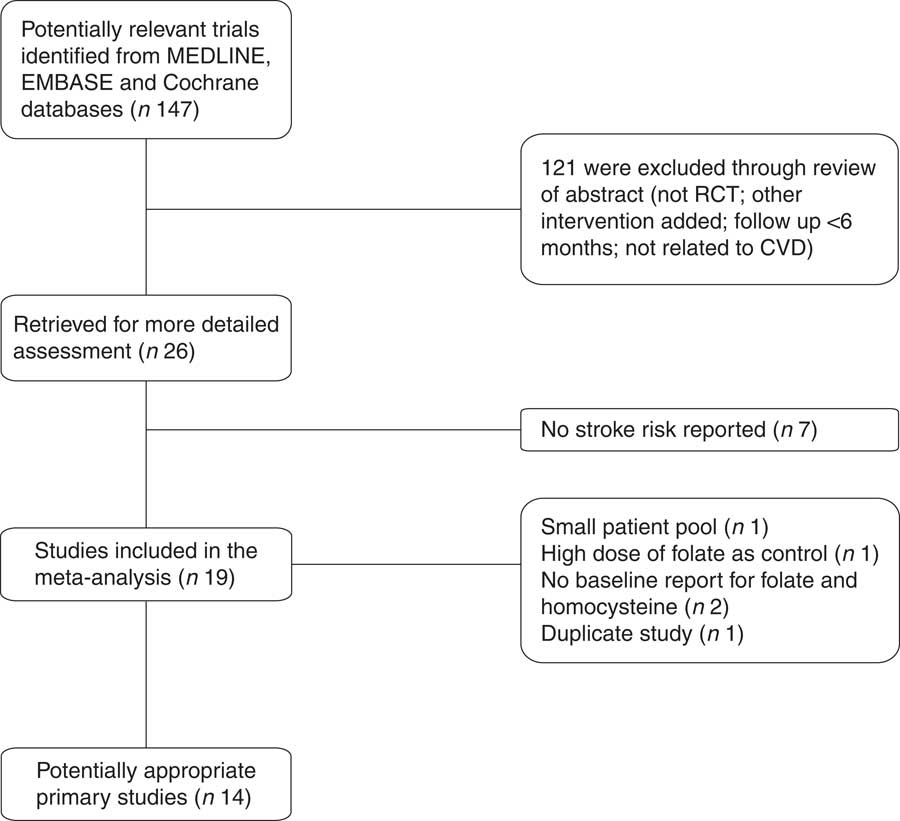
Fig. 1 Flowchart of the search process (RCT, randomized controlled trial)
Table 1 Key characteristics of the trials included in the present meta-analysis
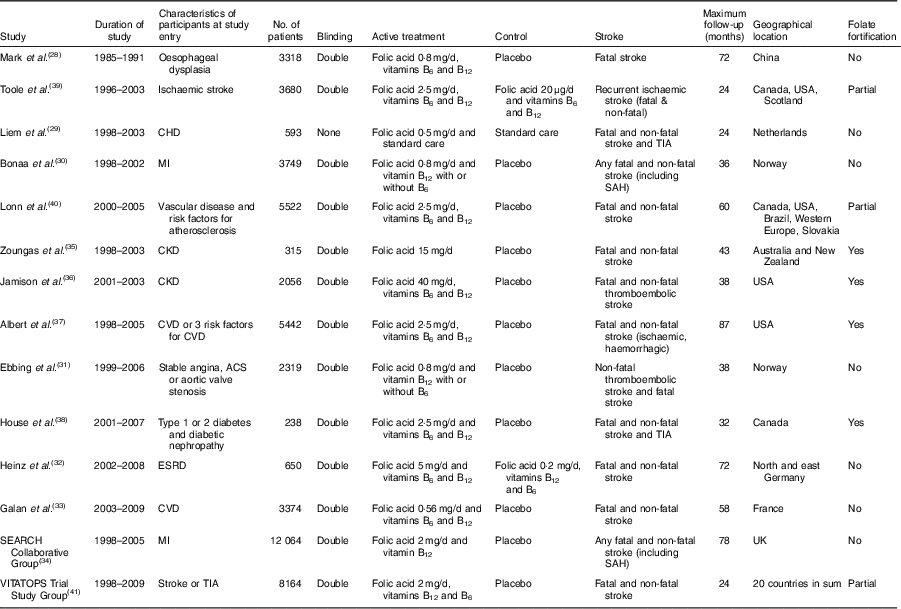
SEARCH, Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine; VITATOPS, VITAmins TO Prevent Stroke; MI, myocardial infarction; CKD, chronic kidney disease; ACS, acute coronary syndrome; ESRD, end-stage renal disease; TIA, transient ischaemic attack; SAH, subarachnoid haemorrhage.
Table 2 Homocysteine change during intervention
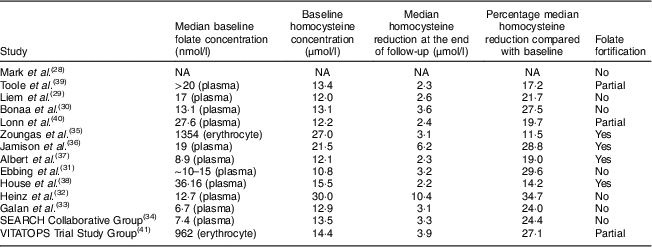
NA, not available.
Homocysteine reductions in the three subgroups were calculated and are presented in Fig. 2. Homocysteine reductions were 26·99 (sd 1·91) %, 18·38 (sd 3·82) % and 21·30 (sd 1·98) %, respectively, in the subgroups without folate fortification, with folate fortification and with partial folate fortification (Fig. 2). Significant difference was observed between the subgroups with folate fortification and without folate fortification (P=0·05), but no significant difference was observed between the subgroups with folate fortification and partial folate fortification (P=0·63) or between the subgroups with partial folate fortification and without folate fortification (P=0·14; Fig. 2).
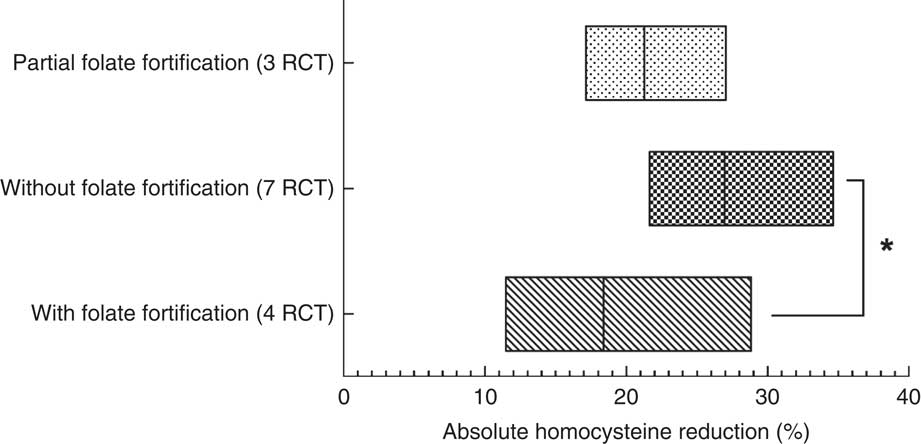
Fig. 2 The level of homocysteine reduction at the end of follow-up among fourteen randomized controlled trials (RCT), with a total of 39 420 patients, studying the effect of folic acid supplementation on stroke risk, with trials stratified according to the status of folate fortification. The absolute mean homocysteine reduction in the active treatment group in the three different folate fortification subgroups is shown; the floating bars depict minimum to maximum, with a line at the mean. Significant difference: *P≤0·05
Figures 3 and 4 show the pooled RR of stroke in the three subgroups. Heterogeneity test showed no significant heterogeneity in the without folate fortification subgroup (P=0·42, I 2=1 %) and the partial folate fortification subgroup (P=0·15, I 2=47 %; Fig. 3), but found significant heterogeneity in the subgroup with folate fortification (P=0·05, I 2=61 %; Fig. 4). Thus, a fixed-effects model was applied to make estimates in the former two subgroups and a random-effects model was used in the subgroup with folate fortification. The RR of stroke was 0·88 (95 % CI 0·77, 1·00, P=0·05) in the subgroup without folate fortification, 0·91 (95 % CI 0·82, 1·01, P=0·09) in the subgroup with partial folate fortification (Fig. 3) and 0·94 (95 % CI 0·58, 1·54, P=0·82) in the subgroup with folate fortification (Fig. 4). Sensitivity analysis found that exclusion of any single study from the analysis did not alter the overall findings for folic acid supplementation on stroke risk. The estimate from the fixed-effects model was similar to the estimate from the random-effects model, suggesting that a small-study effect on the overall intervention effect estimate was not significant.
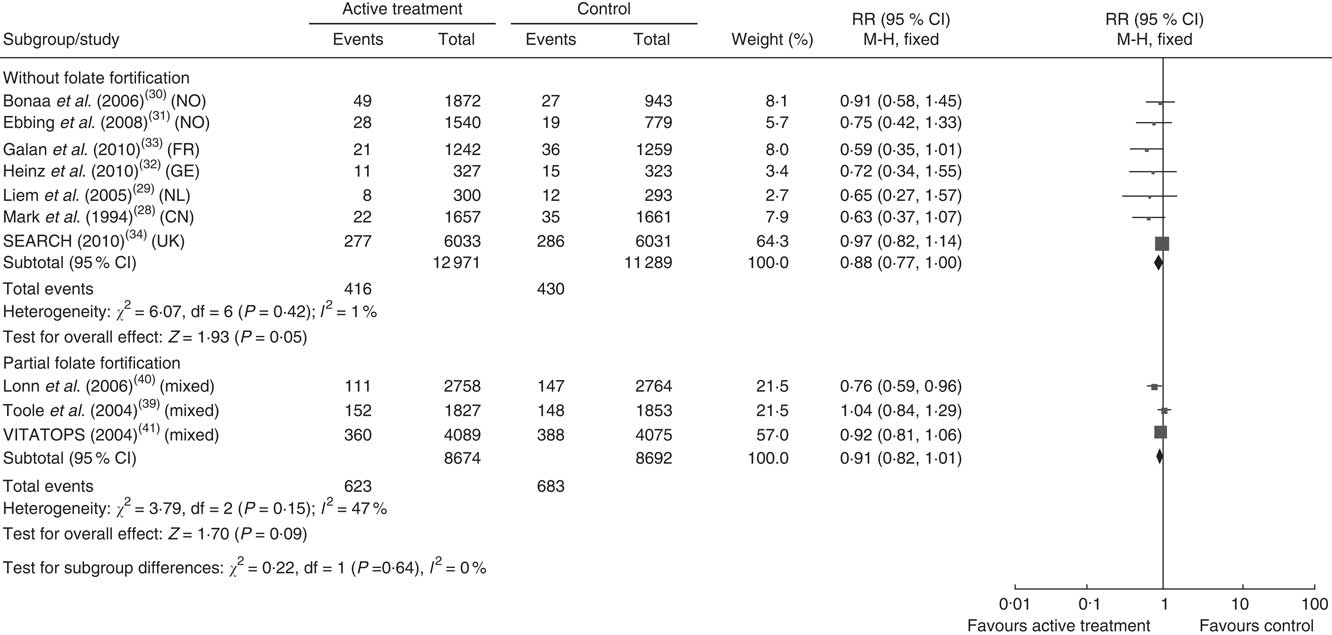
Fig. 3 Pooled relative risk (RR) of stroke in the subgroups without folate fortification and with partial folate fortification. The study-specific RR and 95 % CI are represented by the black square and horizontal line, respectively; the area of the black square is proportional to the specific-study weight to the overall meta-analysis. The centre of the black diamond presents the pooled RR risk and its width represents the pooled 95 % CI (NO, Norway; FR, France; GE, Germany; NL, Netherlands; CN, China)
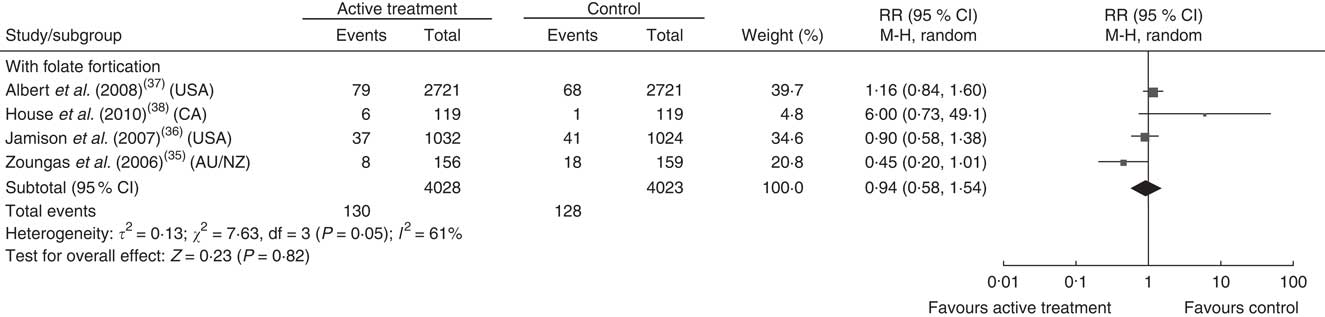
Fig. 4 Pooled relative risk (RR) of stroke in the subgroup with folate fortification. The study-specific RR and 95 % CI are represented by the black square and horizontal line, respectively; the area of the black square is proportional to the specific-study weight to the overall meta-analysis. The centre of the black diamond presents the pooled RR risk and its width represents the pooled 95 % CI (CA, Canada; AU/NZ, Australia/New Zealand)
Discussion
The present meta-analysis included fourteen RCT with a total of 39 420 patients to study the effect of folic acid supplementation on stroke risk by stratifying trials according to the status of folate fortification. It was found that homocysteine reduction was significantly higher in the regions without folate fortification than in the regions with fortification. In addition, folic acid supplementation could significantly reduce the risk of stroke in the regions without folate fortification. Although mild benefit was observed in the subgroup with partial folate fortification, it did not reach a statistically significant level. The main difference between the present compared with previous meta-analyses is the stratification of trials according to the status of folate fortification.
Homocysteine is a risk factor of cerebral small-vessel disease( Reference Hassan, Hunt and O’Sullivan 42 – Reference Tseng, Chang and Liu 44 ). In the present study, the significantly higher homocysteine lowering observed in the subgroup without folate fortification was associated with a significantly lowered risk of stroke events. It is possible that the benefits of homocysteine lowering might be related to the reduced cerebral small-vessel diseases contributing to both ischaemic and haemorrhagic stroke. Shirodaria et al.’s physiological study found that low-dose folic acid supplementation (0·4 mg/d) was sufficient to improve vascular endothelial function and no additional benefit was achieved through increasing the dose to 5 mg/d( Reference Shirodaria, Antoniades and Lee 45 ). After folate fortification in the USA and Canada, stroke mortality decreased slightly( Reference Yang, Botto and Erickson 46 ). This helps to explain why lower stroke risk due to folic acid supplementation was observed in the subgroup without folate fortification but not in the subgroup with folate fortification in the present study. Previous studies reported that through inhibiting hepatic synthesis of apo A1, a major HDL apolipoprotein, homocysteine could reduce serum concentration of HDL cholesterol( Reference Liao, Tan and Hui 47 ). Therefore, homocysteine-induced apo A1 synthesis inhibition is a possible mechanism of atherosclerosis development( Reference Choy, Mymin and Zhu 48 , Reference Ma, Lv and Liu 49 ). Low serum concentration of HDL cholesterol has been considered a predictive factor of stroke and a target for treatment( Reference Sanossian, Saver and Navab 50 ). However, the trials involved in the present meta-analysis all recruited patients with high cardiovascular risk. It is highly possible they had already received intensive lipid profile interventions before folic acid supplementation. Therefore, the effect of homocysteine lowering on HDL cholesterol was difficult to demonstrate in these trials. However, for populations with middle to high risk of cerebrovascular events and without proper medication for lowering lipids, the effect of folate might be more evident. Holmes et al.( Reference Holmes, Newcombe and Hubacek 8 ) observed that in regions with increasing levels of folate or with established policies of population folate fortification, the effect of folic acid-based homocysteine-lowering interventions was not evident in reducing genetic variance-associated stroke risk. The present study also implies the possible benefit is more achievable in the regions without folate fortification.
Some recent studies have suggested that homocysteine is not a primary regulator of endothelial function. Lieb et al. studied the pathophysiological processes of arterial stiffness, which might contribute to atherosclerosis. They observed that homocysteine level was not related to any surrogate biomarkers for arterial stiffness( Reference Lieb, Larson and Benjamin 51 ). Menon et al. studied hyperhomocystinaemia in patients with moderate chronic kidney disease and also found it was not a risk factor for all-cause or cardiovascular mortality after adjustment for kidney function( Reference Menon, Sarnak and Greene 52 ). In fact, homocysteine’s role in disease development might be more evident at early stage than at later stages. Hodis et al.( Reference Hodis, Mack and Dustin 16 ) observed that B-vitamin supplementation could significantly reduce carotid artery intima media thickness and thus lower the progression of early-stage subclinical atherosclerosis. However, this effect was not observed during progression of aortic or coronary artery calcification, which indicates late-stage atherosclerosis. This finding suggests that homocysteine lowering might be effective at early stages of vascular disease. Serum/plasma or erythrocyte concentrations of folate provide an accurate picture of a population’s folate status. According to WHO, the cut-off for defining folate deficiencies based on metabolic indicators are: <10 nmol/l (4·4 ng/ml) for serum folate or <305 nmol/l (140 ng/ml) for erythrocyte folate( 53 ). Based on findings of the present study, it is recommended that for patients with high stroke risk (such as patients with chronic vascular disease, diabetes and/or high blood cholesterol or TAG) in folate-deficient regions, folic acid supplementation could be considered.
The major limitation of the present study is the variance of the trials in respect to patient baseline characteristics, intensity of treatment, duration, primary/secondary end points and other design features. For example, the total baseline serum homocysteine level varied from 11 μmol/l to over 30 μmol/l in the fourteen trials involved. Since previous prospective studies indicated that patients with homocysteine above 10·2 μmol/l carried doubled risk of stroke and 20 μmol/l was associated with ninefold higher stroke risk( Reference von Eckardstein and Assmann 54 , Reference Graham, Daly and Refsum 55 ), it is reasonable that homocysteine-lowering interventions might achieve a better stroke preventive effect in patients with high homocysteine. This might lead to selection bias in the present study. However, sensitivity testing confirmed the robustness of the findings.
Conclusion
To sum up, the present meta-analysis found a significant benefit of folic acid supplementation in reducing stroke risk in regions without folate fortification. Homocysteine lowering was also significantly higher in these regions compared with the regions with fortification. Since folic acid supplementation is a proven safe, inexpensive and easy-to-manipulate intervention, it could be recommended to patients with high risk of stroke in regions without folate fortification.
Acknowledgements
Financial support: This study was supported by the National Natural Science Foundation of China (grant number 81200153) and the Science Foundation of Health Department, Sichuan Province (grant number 120213). The funders had no role in the design, analysis or writing of this article. Conflict of interest: None. Authorship: R.Z. and C.-H.X. contributed equally to the study. R.Z. carried out research design, data collection, data analysis, manuscript preparation and manuscript revision. C.-H.X. carried out data collection and data analysis. Y.-N.X. carried out research design and manuscript revision. Y.-L.W. carried out data analysis and manuscript preparation. M.W. carried out research design and applying for grants. Ethics of human subject participation: Ethical approval was not required.










