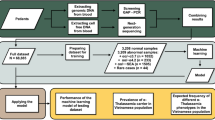
Abstract
Currently, amniocentesis, chorionic villus sampling (CVS) and fetal blood sampling are used to obtain fetal cells for genetic diagnosis. These invasive procedures pose a small but not negligible risk for the fetus. Efforts have been directed towards the enrichment of fetal cells, such as erythroblasts, from maternal blood and progress has been made in the diagnosis of some chromosomal disorders and in sex determinations. We now report the detection of point mutations in single gene disorders using this method of prenatal diagnosis by enriching fetal cells from maternal blood by magnetic cell sorting followed by isolation of pure fetal cells by microdissection. In two pregnancies at risk for sickle cell anaemia and β–thalassaemia, we successfully identified the fetal genotypes. Thus, prenatal diagnosis of single gene disorders by recovering fetal cells from maternal circulation appears to be a feasible approach.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Kan, Y.W., Golbus, M.S., Klein, P. & Dozy, A.M. Successful application of prenatal diagnosis in a pregnancy at risk for homozygous β-thalassemia. New Engl. J. Med. 292, 1096–1099 (1975).
Kan, Y.W. & Golbus, M.S. and Trecartin, R. Prenatal diagnosis of sickle cell anemia. New Engl. J. Med. 294, 1039–1040 (1976).
Ottolenghi, S. et al. The severe form of alpha thalassaemia is caused by a haemoglobin gene deletion. Nature 251, 389–391 (1974).
Taylor, J.M. et al. Genetic lesion in homozygous α-thalassaemia (hydrops fetalis). Nature 251, 392–393 (1974).
Kan, Y.W., Golbus, M.S. & Dozy, A.M. Prenatal diagnosis of α-thalassemia: Clinical application of molecular hybridization. New Engl. J. Med. 295, 1165–1167 (1976).
Dozy, A.M. et al. Prenatal diagnosis of homozygous α thalassemia. J. Am. Med. Assoc. 241, 1610–1612 (1979).
Kan, Y.W. & Dozy, A.M. Polymorphism of DNA sequence adjacent to human β-globin structural gene: Relationship to sickle mutation. Proc. Natl. Acad. Sci. USA 75, 5631–5635 (1978).
Chang, J.C. & Kan, Y.W. A sensitive new prenatal test for sickle cell anemia. New Engl. J. Med. 307, 30–32 (1982).
Orkin, S.H., Little, P.F.R., Kazazian, H.H. Jr., & Brehm, C.D. Improved detection of the sickle mutation by DNA analysis and its application to prenatal diagnosis. New Engl. J. Med. 307, 32–36 (1982).
Pirastu, M. et al. Prenatal diagnosis of β thalassemia: Direct detection of a single nucleotide mutation in DNA. New Engl. J. Med. 309, 284–287 (1983).
Saiki, R.K. et al. Enzymatic amplification of β-globin genome sequences and restriction site analysis for diagnosis of sickle cell anemia. Science 230, 1350–1352 (1985).
Evans, M.I. et al. Genetic diagnosis in the first trimester: the norm for the 1990s. Am. J. Obstet. Gynec. 160, 1332–1339 (1989).
Simpson, J.L., Elias, S. Isolating fetal cells from maternal blood: Advances in prenatal diagnosis through molecular technology. J. Am. Med. Assoc. 270, 2357–2361 (1993).
Adinolfi, M. Non-or minimally invasive prenatal diagnostic tests on maternal blood samples or transcervical cells. Prenat. Diagn. 15, 889–896 (1995).
Bianchi, D. Prenatal diagnosis by analysis of fetal cells in maternal blood. J. Pediatr. 127, 847–856 (1995).
Thomas, M.R. et al. The time of appearance and disappearance of fetal DNA from the maternal circulation. Prenat. Diagn. 15, 641–646 (1995).
Herzenberg, L.A., Bianchi, D.W., Shroder, J., Cann, H.M., Iverson, G.M. Fetal cells in the blood of pregnant women: Detection and enrichment by fluorescence-activated cell sorting. Proc. Natl. Acad. Sci. USA 76, 1453–1455 (1979).
Schröder;, J., Tiilikainen, A. & de la Chapelle, A. Fetal leukocytes in the maternal circulation after delivery. Transplantation 17, 346–354 (1974).
Bianchi, D.W., Zickwolf, G.K., Weil, G.J., Sylvester, S. & DeMaria, M.A. Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum. Proc. Natl. Acad. Sci. USA 93, 705–708 (1996).
Hawes, C. et al. Detection of paternally inherited mutations for β-thalassemia in trophoblasts isolated from peripheral maternal blood. Ann. N. Y. Acad. Sci. 731, 217–225 (1994).
Adinolfi, M. et al. Detection of fetal cells in transcervical samples and prenatal diagnosis of chromosomal abnormalities. Prenat. Diagn. 15, 943–949 (1995).
Tutschek, B. et al. Isolation of fetal cells from transcervical samples by micromanipulation: Molecular confirmation of their fetal origin and diagnosis of fetal aneuploidy. Prenat. Diagn. 15, 951–960 (1995).
Bianchi, D.W., Flint, A.F., Pizzimenti, M.F., Knoll, J.H.M. & Latt, S.A. Isolation of fetal DNA from nucleated erythrocytes in maternal blood. Proc. Natl. Acad. Sci. U.S.A. 87, 3279–3283 (1990).
Bianchi, D.W. et al. Erythroid-specific antibodies enhance detection of fetal nucleated erythrocytes in maternal blood. Prenat. Diagn. 13, 293–300 (1993).
Price, J.O. et al. Prenatal diagnosis with fetal cells isolated from maternal blood by multiparameter flow cytometry. Am. J. Obstet. Gynec. 165, 1731–1737 (1991).
Gänshirt-Ahlert, D. et al. Magnetic cell sorting and the transferrin receptor as potential means of prenatal diagnosis from maternal blood. Am. J. Obstet. Gynec. 166, 1350–1355 (1992).
Gänshirt-Ahlert, D. et al. Detection of fetal trisomies 21 and 18 from maternal blood using triple gradient and magnetic cell sorting. Am. J. Rep. Immunol. 30, 194–201 (1993).
Büsch, J. et al. Enrichment of fetal cells from maternal blood by high gradient magnetic cell sorting (Double MACS) for PCR-based genetic analysis. Prenat. Diagn. 14, 1129–1140 (1994).
Zheng, Y.L. et al. Prenatal diagnosis from maternal blood: Simultaneous immunophenotyping and FISH of fetal nucleated erythrocytes isolated by negative magnetic cell sorting. J. Med. Genet. 30, 1151–1156 (1993).
Zheng, Y.-L., DeMaria, M., Zhen, D., Vadnais, T.J., & Bianchi, D.W. Flow sorting of fetal erythroblasts using intracytoplasmic anti-fetal haemoglobin: Preliminary observation on maternal samples. Prenat. Diagn. 15, 897–905 (1995).
Bianchi, D. et al. Development of a model system to compare cell separation methods for the isolation of fetal cells from maternai blood. Prenat. Diagn. 16, 289–298 (1996).
Geifman-Holtzman, O. et al. Detection of fetal HLA-DQa sequences in maternal blood: A gender-independent technique of fetal cell identification. Prenat. Diagn. 15, 261–268 (1995).
Saiki, R.K. et al. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science 239, 487–491 (1988).
Maggio, A. et al. Rapid and simultaneous typing of hemoglobin S, hemoglobin C, and seven Mediterranean beta-thalassemia mutations by covalent reverse dot-blot analysis: Application to prenatal diagnosis in Sicily. Blood 81, 239–242 (1993).
Cai, S.P., Wall, J., Kan, Y.W. & Chehab, F.F. Reverse dot blot probes for the screening of β-thalassemia mutations in Asians and American blacks. Human Mutation 3, 59–63 (1994).
Chehab, F.F., Wall, J. & Cai, S.P. Analysis of PCR products by covalent reverse dot blot hybridization. in PCR Strategies. 30–139 (Academic Press, Inc. New York, 1995).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cheung, MC., Goldberg, J. & Kan, Y. Prenatal diagnosis of sickle cell anaemia and thalassaemia by analysis of fetal cells in maternal blood. Nat Genet 14, 264–268 (1996). https://doi.org/10.1038/ng1196-264
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ng1196-264
This article is cited by
-
Noninvasive prenatal diagnosis targeting fetal nucleated red blood cells
Journal of Nanobiotechnology (2022)
-
Margination of Stiffened Red Blood Cells Regulated By Vessel Geometry
Scientific Reports (2017)
-
Dielectrophoretic force measurement of red blood cells exposed to oxidative stress using optical tweezers and a microfluidic chip
Biomedical Engineering Letters (2017)
-
Discordant circulating fetal DNA and subsequent cytogenetics reveal false negative, placental mosaic, and fetal mosaic cfDNA genotypes
Journal of Translational Medicine (2015)