Abstract
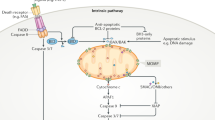
Mitochondria have been classically characterized as organelles with responsibility for cellular energy production in the form of ATP, but they are also the organelles through which apoptotic signaling occurs. Cell stress stimuli can result in outer membrane permeabilization, after which mitochondria release numerous proteins involved in apoptotic signaling, including cytochrome c, apoptosis-inducing factor, endonuclease G, Smac/DIABLO and Omi/HtrA2. Cell fate is determined by signaling through apoptotic proteins within the Bcl-2 (B-cell lymphoma 2) protein family, which converges on mitochondria. Many cancerous cells display abnormal levels of Bcl-2 protein family member expression that results in defective apoptotic signaling. Alterations in bioenergetic function also contribute to cancer as well as numerous other disorders. Recent evidence indicates that several pro-apoptotic proteins localized within mitochondria, as well as proteins within the Bcl-2 protein family, can influence mitochondrial bioenergetic function. This review focuses on the emerging roles of these proteins in the control of mitochondrial activity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Vender Heiden MG, Cantley LC, Thompson CB . Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 2009; 324: 1029–1033.
Metallo CM, Gameiro PA, Bell EL, Mattaini KR, Yang J, Hiller K et al. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature 2011; 481: 380–384.
Mullen AR, Wheaton WW, Jin ES, Chen PH, Sullivan LB, Cheng T et al. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature 2011; 481: 385–388.
Stock D, Leslie AG, Walker JE . Molecular architecture of the rotary motor in ATP synthase. Science 1999; 286: 1700–1705.
Andrews ZB, Diano S, Horvath TL . Mitochondrial uncoupling proteins in the CNS: in support of function and survival. Nat Rev Neurosci 2005; 6: 829–840.
Nicholls DG, Budd SL . Mitochondria and neuronal survival. Physiol Rev 2000; 80: 315–360.
Lemasters JJ, Holmuhamedov E . Voltage-dependent anion channel (VDAC) as mitochondrial governator--thinking outside the box. Biochim Biophys Acta 2006; 1762: 181–190.
Cadenas E, Davies KJ . Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med 2000; 29: 222–230.
Han D, Williams E, Cadenas E . Mitochondrial respiratory chain-dependent generation of superoxide anion and its release into the intermembrane space. Biochem J 2001; 353: 411–416.
Turrens JF, Alexandre A, Lehninger AL . Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Arch Biochem Biophys 1985; 237: 408–414.
Youdim MB, Edmondson D, Tipton KF . The therapeutic potential of monoamine oxidase inhibitors. Nat Rev Neurosci 2006; 7: 295–309.
Mammucari C, Rizzuto R . Signaling pathways in mitochondrial dysfunction and aging. Mech Ageing Dev 2010; 131: 536–543.
Clement MV, Pervaiz S . Intracellular superoxide and hydrogen peroxide concentrations: a critical balance that determines survival or death. Redox Rep 2001; 6: 211–214.
Krishna S, Low IC, Pervaiz S . Regulation of mitochondrial metabolism: yet another facet in the biology of the oncoprotein Bcl-2. Biochem J 2011; 435: 545–551.
Youle RJ, Strasser A . The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 2008; 9: 47–59.
Tait SW, Green DR . Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol 2010; 11: 621–632.
Kroemer G, Martin SJ . Caspase-independent cell death. Nat Med 2005; 11: 725–730.
Hanahan D, Weinberg RA . Hallmarks of cancer: the next generation. Cell 2011; 144: 646–674.
Adams JM, Cory S . The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene 2007; 26: 1324–1337.
Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 2001; 292: 727–730.
Brunelle JK, Letai A . Control of mitochondrial apoptosis by the Bcl-2 family. J Cell Sci 2009; 122: 437–441.
Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ . Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell 2002; 2: 183–192.
Ni Chonghaile T, Sarosiek KA, Vo TT, Ryan JA, Tammareddi A, Moore Vdel G et al. Pretreatment mitochondrial priming correlates with clinical response to cytotoxic chemotherapy. Science 2012; 334: 1129–1133.
Weisova P, Davila D, Tuffy LP, Ward MW, Concannon CG, Prehn JH . Role of 5'-adenosine monophosphate-activated protein kinase in cell survival and death responses in neurons. Antioxid Redox Signal 2010; 14: 1863–1876.
Hardie DG . AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev 2011; 25: 1895–1908.
Jeon SM, Chandel NS, Hay N . AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature 2012; 485: 661–665.
Kato K, Ogura T, Kishimoto A, Minegishi Y, Nakajima N, Miyazaki M et al. Critical roles of AMP-activated protein kinase in constitutive tolerance of cancer cells to nutrient deprivation and tumor formation. Oncogene 2002; 21: 6082–6090.
Laderoute KR, Amin K, Calaoagan JM, Knapp M, Le T, Orduna J et al. 5'-AMP-activated protein kinase (AMPK) is induced by low-oxygen and glucose deprivation conditions found in solid-tumor microenvironments. Mol Cell Biol 2006; 26: 5336–5347.
Concannon CG, Tuffy LP, Weisova P, Bonner HP, Davila D, Bonner C et al. AMP kinase-mediated activation of the BH3-only protein Bim couples energy depletion to stress-induced apoptosis. J Cell Biol 2010; 189: 83–94.
Kilbride SM, Farrelly AM, Bonner C, Ward MW, Nyhan KC, Concannon CG et al. AMP-activated protein kinase mediates apoptosis in response to bioenergetic stress through activation of the pro-apoptotic Bcl-2 homology domain-3-only protein BMF. J Biol Chem 2010; 285: 36199–36206.
Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD . Metformin and reduced risk of cancer in diabetic patients. BMJ 2005; 330: 1304–1305.
Decensi A, Puntoni M, Goodwin P, Cazzaniga M, Gennari A, Bonanni B et al. Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev Res 2010; 3: 1451–1461.
Zamzami N, Larochette N, Kroemer G . Mitochondrial permeability transition in apoptosis and necrosis. Cell Death Differ 2005; 12 (Suppl 2): 1478–1480.
Dong XX, Wang Y, Qin ZH . Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacol Sin 2009; 30: 379–387.
Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 2005; 434: 658–662.
Kokoszka JE, Waymire KG, Levy SE, Sligh JE, Cai J, Jones DP et al. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature 2004; 427: 461–465.
Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H et al. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 2005; 434: 652–658.
Shulga N, Wilson-Smith R, Pastorino JG . Sirtuin-3 deacetylation of cyclophilin D induces dissociation of hexokinase II from the mitochondria. J Cell Sci 2010; 123: 894–902.
Pedersen PL . Warburg, me and Hexokinase 2: Multiple discoveries of key molecular events underlying one of cancers’ most common phenotypes, the “Warburg Effect”, i.e., elevated glycolysis in the presence of oxygen. J Bioenerg Biomembr 2007; 39: 211–222.
Abu-Hamad S, Zaid H, Israelson A, Nahon E, Shoshan-Barmatz V . Hexokinase-I protection against apoptotic cell death is mediated via interaction with the voltage-dependent anion channel-1: mapping the site of binding. J Biol Chem 2008; 283: 13482–13490.
Belzacq AS, Vieira HL, Verrier F, Vandecasteele G, Cohen I, Prevost MC et al. Bcl-2 and Bax modulate adenine nucleotide translocase activity. Cancer Res 2003; 63: 541–546.
Marzo I, Brenner C, Zamzami N, Susin SA, Beutner G, Brdiczka D et al. The permeability transition pore complex: a target for apoptosis regulation by caspases and bcl-2-related proteins. J Exp Med 1998; 187: 1261–1271.
Shimizu S, Eguchi Y, Kamiike W, Funahashi Y, Mignon A, Lacronique V et al. Bcl-2 prevents apoptotic mitochondrial dysfunction by regulating proton flux. Proc Natl Acad Sci USA 1998; 95: 1455–1459.
Tsujimoto Y, Nakagawa T, Shimizu S . Mitochondrial membrane permeability transition and cell death. Biochim Biophys Acta 2006; 1757: 1297–1300.
Hosler JP, Ferguson-Miller S, Mills DA . Energy transduction: proton transfer through the respiratory complexes. Annu Rev Biochem 2006; 75: 165–187.
Li K, Li Y, Shelton JM, Richardson JA, Spencer E, Chen ZJ et al. Cytochrome c deficiency causes embryonic lethality and attenuates stress-induced apoptosis. Cell 2000; 101: 389–399.
Vempati UD, Han X, Moraes CT . Lack of cytochrome c in mouse fibroblasts disrupts assembly/stability of respiratory complexes I and IV. J Biol Chem 2009; 284: 4383–4391.
Garrido C, Galluzzi L, Brunet M, Puig PE, Didelot C, Kroemer G . Mechanisms of cytochrome c release from mitochondria. Cell Death Differ 2006; 13: 1423–1433.
Liu X, Kim CN, Yang J, Jemmerson R, Wang X . Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 1996; 86: 147–157.
Zou H, Henzel WJ, Liu X, Lutschg A, Wang X . Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell 1997; 90: 405–413.
Hao Z, Duncan GS, Chang CC, Elia A, Fang M, Wakeham A et al. Specific ablation of the apoptotic functions of cytochrome C reveals a differential requirement for cytochrome C and Apaf-1 in apoptosis. Cell 2005; 121: 579–591.
Cecconi F, Alvarez-Bolado G, Meyer BI, Roth KA, Gruss P . Apaf1 (CED-4 homolog) regulates programmed cell death in mammalian development. Cell 1998; 94: 727–737.
Kuida K, Haydar TF, Kuan CY, Gu Y, Taya C, Karasuyama H et al. Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9. Cell 1998; 94: 325–337.
Waterhouse NJ, Sedelies KA, Sutton VR, Pinkoski MJ, Thia KY, Johnstone R et al. Functional dissociation of DeltaPsim and cytochrome c release defines the contribution of mitochondria upstream of caspase activation during granzyme B-induced apoptosis. Cell Death Differ 2006; 13: 607–618.
Waterhouse NJ, Goldstein JC, von Ahsen O, Schuler M, Newmeyer DD, Green DR . Cytochrome c maintains mitochondrial transmembrane potential and ATP generation after outer mitochondrial membrane permeabilization during the apoptotic process. J Cell Biol 2001; 153: 319–328.
Mootha VK, Wei MC, Buttle KF, Scorrano L, Panoutsakopoulou V, Mannella CA et al. A reversible component of mitochondrial respiratory dysfunction in apoptosis can be rescued by exogenous cytochrome c. EMBO J 2001; 20: 661–671.
Dussmann H, Rehm M, Kogel D, Prehn JH . Outer mitochondrial membrane permeabilization during apoptosis triggers caspase-independent mitochondrial and caspase-dependent plasma membrane potential depolarization: a single-cell analysis. J Cell Sci 2003; 116: 525–536.
Rego AC, Vesce S, Nicholls DG . The mechanism of mitochondrial membrane potential retention following release of cytochrome c in apoptotic GT1-7 neural cells. Cell Death Differ 2001; 8: 995–1003.
Huber HJ, Dussmann H, Kilbride SM, Rehm M, Prehn JH . Glucose metabolism determines resistance of cancer cells to bioenergetic crisis after cytochrome-c release. Mol Syst Biol 2011; 7: 470.
Susin SA, Zamzami N, Castedo M, Hirsch T, Marchetti P, Macho A et al. Bcl-2 inhibits the mitochondrial release of an apoptogenic protease. J Exp Med 1996; 184: 1331–1341.
Zamzami N, Susin SA, Marchetti P, Hirsch T, Gomez-Monterrey I, Castedo M et al. Mitochondrial control of nuclear apoptosis. J Exp Med 1996; 183: 1533–1544.
Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 1999; 397: 441–446.
Vahsen N, Cande C, Briere JJ, Benit P, Joza N, Larochette N et al. AIF deficiency compromises oxidative phosphorylation. EMBO J 2004; 23: 4679–4689.
Cande C, Vahsen N, Garrido C, Kroemer G . Apoptosis-inducing factor (AIF): caspase-independent after all. Cell Death Differ 2004; 11: 591–595.
Brown D, Yu BD, Joza N, Benit P, Meneses J, Firpo M et al. Loss of Aif function causes cell death in the mouse embryo, but the temporal progression of patterning is normal. Proc Natl Acad Sci USA 2006; 103: 9918–9923.
Joza N, Oudit GY, Brown D, Benit P, Kassiri Z, Vahsen N et al. Muscle-specific loss of apoptosis-inducing factor leads to mitochondrial dysfunction, skeletal muscle atrophy, and dilated cardiomyopathy. Mol Cell Biol 2005; 25: 10261–10272.
Klein JA, Longo-Guess CM, Rossmann MP, Seburn KL, Hurd RE, Frankel WN et al. The harlequin mouse mutation downregulates apoptosis-inducing factor. Nature 2002; 419: 367–374.
Cheung EC, Joza N, Steenaart NA, McClellan KA, Neuspiel M, McNamara S et al. Dissociating the dual roles of apoptosis-inducing factor in maintaining mitochondrial structure and apoptosis. EMBO J 2006; 25: 4061–4073.
Miramar MD, Costantini P, Ravagnan L, Saraiva LM, Haouzi D, Brothers G et al. NADH oxidase activity of mitochondrial apoptosis-inducing factor. J Biol Chem 2001; 276: 16391–16398.
van Empel VP, Bertrand AT, van der Nagel R, Kostin S, Doevendans PA, Crijns HJ et al. Downregulation of apoptosis-inducing factor in harlequin mutant mice sensitizes the myocardium to oxidative stress-related cell death and pressure overload-induced decompensation. Circ Res 2005; 96: e92–e101.
Krantic S, Mechawar N, Reix S, Quirion R . Apoptosis-inducing factor: a matter of neuron life and death. Prog Neurobiol 2007; 81: 179–196.
Chinta SJ, Rane A, Yadava N, Andersen JK, Nicholls DG, Polster BM . Reactive oxygen species regulation by AIF- and complex I-depleted brain mitochondria. Free Radic Biol Med 2009; 46: 939–947.
Ohsato T, Ishihara N, Muta T, Umeda S, Ikeda S, Mihara K et al. Mammalian mitochondrial endonuclease G. Digestion of R-loops and localization in intermembrane space. Eur J Biochem 2002; 269: 5765–5770.
Uren RT, Dewson G, Bonzon C, Lithgow T, Newmeyer DD, Kluck RM . Mitochondrial release of pro-apoptotic proteins: electrostatic interactions can hold cytochrome c but not Smac/DIABLO to mitochondrial membranes. J Biol Chem 2005; 280: 2266–2274.
Li LY, Luo X, Wang X . Endonuclease G is an apoptotic DNase when released from mitochondria. Nature 2001; 412: 95–99.
Zanna C, Ghelli A, Porcelli AM, Martinuzzi A, Carelli V, Rugolo M . Caspase-independent death of Leber's hereditary optic neuropathy cybrids is driven by energetic failure and mediated by AIF and Endonuclease G. Apoptosis 2005; 10: 997–1007.
Wu Y, Dong M, Toepfer NJ, Fan Y, Xu M, Zhang J . Role of endonuclease G in neuronal excitotoxicity in mice. Neurosci Lett 2004; 364: 203–207.
Basnakian AG, Apostolov EO, Yin X, Abiri SO, Stewart AG, Singh AB et al. Endonuclease G promotes cell death of non-invasive human breast cancer cells. Exp Cell Res 2006; 312: 4139–4149.
Schafer P, Scholz SR, Gimadutdinow O, Cymerman IA, Bujnicki JM, Ruiz-Carrillo A et al. Structural and functional characterization of mitochondrial EndoG, a sugar non-specific nuclease which plays an important role during apoptosis. J Mol Biol 2004; 338: 217–228.
Ishihara Y, Shimamoto N . Involvement of endonuclease G in nucleosomal DNA fragmentation under sustained endogenous oxidative stress. J Biol Chem 2006; 281: 6726–6733.
McDermott-Roe C, Ye J, Ahmed R, Sun XM, Serafin A, Ware J et al. Endonuclease G is a novel determinant of cardiac hypertrophy and mitochondrial function. Nature 2011; 478: 114–118.
Cote J, Ruiz-Carrillo A . Primers for mitochondrial DNA replication generated by endonuclease G. Science 1993; 261: 765–769.
Suzuki Y, Imai Y, Nakayama H, Takahashi K, Takio K, Takahashi R . A serine protease, HtrA2, is released from the mitochondria and interacts with XIAP, inducing cell death. Mol Cell 2001; 8: 613–621.
Yang QH, Church-Hajduk R, Ren J, Newton ML, Du C . Omi/HtrA2 catalytic cleavage of inhibitor of apoptosis (IAP) irreversibly inactivates IAPs and facilitates caspase activity in apoptosis. Genes Dev 2003; 17: 1487–1496.
Vande Walle L, Van Damme P, Lamkanfi M, Saelens X, Vandekerckhove J, Gevaert K et al. Proteome-wide identification of HtrA2/Omi substrates. J Proteome Res 2007; 6: 1006–1015.
Jones JM, Datta P, Srinivasula SM, Ji W, Gupta S, Zhang Z et al. Loss of Omi mitochondrial protease activity causes the neuromuscular disorder of mnd2 mutant mice. Nature 2003; 425: 721–727.
Martins LM, Morrison A, Klupsch K, Fedele V, Moisoi N, Teismann P et al. Neuroprotective role of the Reaper-related serine protease HtrA2/Omi revealed by targeted deletion in mice. Mol Cell Biol 2004; 24: 9848–9862.
Strauss KM, Martins LM, Plun-Favreau H, Marx FP, Kautzmann S, Berg D et al. Loss of function mutations in the gene encoding Omi/HtrA2 in Parkinson’s disease. Hum Mol Genet 2005; 14: 2099–2111.
Du C, Fang M, Li Y, Li L, Wang X . Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 2000; 102: 33–42.
Verhagen AM, Ekert PG, Pakusch M, Silke J, Connolly LM, Reid GE et al. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell 2000; 102: 43–53.
Wu G, Chai J, Suber TL, Wu JW, Du C, Wang X et al. Structural basis of IAP recognition by Smac/DIABLO. Nature 2000; 408: 1008–1012.
Okada H, Suh WK, Jin J, Woo M, Du C, Elia A et al. Generation and characterization of Smac/DIABLO-deficient mice. Mol Cell Biol 2002; 22: 3509–3517.
Cheng J, Zhu Y, He S, Lu Y, Chen J, Han B et al. Functional mutation of SMAC/DIABLO, encoding a mitochondrial proapoptotic protein, causes human progressive hearing loss DFNA64. Am J Hum Genet 2011; 89: 56–66.
Flanagan L, Sebastia J, Tuffy LP, Spring A, Lichawska A, Devocelle M et al. XIAP impairs Smac release from the mitochondria during apoptosis. Cell Death Dis 2010; 1: e49.
Hsu YT, Wolter KG, Youle RJ . Cytosol-to-membrane redistribution of Bax and Bcl-X(L) during apoptosis. Proc Natl Acad Sci USA 1997; 94: 3668–3672.
Burge D, Youle L, McIntosh N . An audit of transfers for neonatal surgical care in England in 2007. Arch Dis Child Fetal Neonatal Ed 2009; 94: F290–F293.
Whelan RS, Konstantinidis K, Wei AC, Chen Y, Reyna DE, Jha S et al. Bax regulates primary necrosis through mitochondrial dynamics. Proc Natl Acad Sci USA 2012; 109: 6566–6571.
Jones RG, Bui T, White C, Madesh M, Krawczyk CM, Lindsten T et al. The proapoptotic factors Bax and Bak regulate T Cell proliferation through control of endoplasmic reticulum Ca(2+) homeostasis. Immunity 2007; 27: 268–280.
Karbowski J, Cronin CJ, Seah A, Mendel JE, Cleary D, Sternberg PW . Conservation rules, their breakdown, and optimality in Caenorhabditis sinusoidal locomotion. J Theor Biol 2006; 242: 652–669.
Boohaker RJ, Zhang G, Carlson AL, Nemec KN, Khaled AR . BAX supports the mitochondrial network, promoting bioenergetics in nonapoptotic cells. Am J Physiol Cell Physiol 2011; 300: C1466–C1478.
Pegoraro L, Palumbo A, Erikson J, Falda M, Giovanazzo B, Emanuel BS et al. A 14;18 and an 8;14 chromosome translocation in a cell line derived from an acute B-cell leukemia. Proc Natl Acad Sci USA 1984; 81: 7166–7170.
Chipuk JE, Bouchier-Hayes L, Green DR . Mitochondrial outer membrane permeabilization during apoptosis: the innocent bystander scenario. Cell Death Differ 2006; 13: 1396–1402.
Akao Y, Otsuki Y, Kataoka S, Ito Y, Tsujimoto Y . Multiple subcellular localization of bcl-2: detection in nuclear outer membrane, endoplasmic reticulum membrane, and mitochondrial membranes. Cancer Res 1994; 54: 2468–2471.
Chen ZX, Pervaiz S . Bcl-2 induces pro-oxidant state by engaging mitochondrial respiration in tumor cells. Cell Death Differ 2007; 14: 1617–1627.
Chen ZX, Pervaiz S . Involvement of cytochrome c oxidase subunits Va and Vb in the regulation of cancer cell metabolism by Bcl-2. Cell Death Differ 2010; 17: 408–420.
Low IC, Kang J, Pervaiz S . Bcl-2: a prime regulator of mitochondrial redox metabolism in cancer cells. Antioxid Redox Signal 2011; 15: 2975–2987.
Boise LH, Gonzalez-Garcia M, Postema CE, Ding L, Lindsten T, Turka LA et al. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell 1993; 74: 597–608.
Motoyama N, Wang F, Roth KA, Sawa H, Nakayama K, Nakayama K et al. Massive cell death of immature hematopoietic cells and neurons in Bcl-x-deficient mice. Science 1995; 267: 1506–1510.
Vander Heiden MG, Li XX, Gottleib E, Hill RB, Thompson CB, Colombini M . Bcl-xL promotes the open configuration of the voltage-dependent anion channel and metabolite passage through the outer mitochondrial membrane. J Biol Chem 2001; 276: 19414–19419.
Karbowski M, Norris KL, Cleland MM, Jeong SY, Youle RJ . Role of Bax and Bak in mitochondrial morphogenesis. Nature 2006; 443: 658–662.
Levine B, Sinha S, Kroemer G . Bcl-2 family members: dual regulators of apoptosis and autophagy. Autophagy 2008; 4: 600–606.
Hickman JA, Hardwick JM, Kaczmarek LK, Jonas EA . Bcl-xL inhibitor ABT-737 reveals a dual role for Bcl-xL in synaptic transmission. J Neurophysiol 2008; 99: 1515–1522.
Alavian KN, Li H, Collis L, Bonanni L, Zeng L, Sacchetti S et al. Bcl-xL regulates metabolic efficiency of neurons through interaction with the mitochondrial F1FO ATP synthase. Nat Cell Biol 2011; 13: 1224–1233.
Chen YB, Aon MA, Hsu YT, Soane L, Teng X, McCaffery JM et al. Bcl-xL regulates mitochondrial energetics by stabilizing the inner membrane potential. J Cell Biol 2011; 195: 263–276.
Jeong SY, Gaume B, Lee YJ, Hsu YT, Ryu SW, Yoon SH et al. Bcl-x(L) sequesters its C-terminal membrane anchor in soluble, cytosolic homodimers. EMBO J 2004; 23: 2146–2155.
Opferman JT, Iwasaki H, Ong CC, Suh H, Mizuno S, Akashi K et al. Obligate role of anti-apoptotic MCL-1 in the survival of hematopoietic stem cells. Science 2005; 307: 1101–1104.
Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ . Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature 2003; 426: 671–676.
Arbour N, Vanderluit JL, Le Grand JN, Jahani-Asl A, Ruzhynsky VA, Cheung EC et al. Mcl-1 is a key regulator of apoptosis during CNS development and after DNA damage. J Neurosci 2008; 28: 6068–6078.
Rinkenberger JL, Horning S, Klocke B, Roth K, Korsmeyer SJ . Mcl-1 deficiency results in peri-implantation embryonic lethality. Genes Dev 2000; 14: 23–27.
Beroukhim R, Mermel CH, Porter D, Wei G, Raychaudhuri S, Donovan J et al. The landscape of somatic copy-number alteration across human cancers. Nature 2010; 463: 899–905.
Wuilleme-Toumi S, Robillard N, Gomez P, Moreau P, Le Gouill S, Avet-Loiseau H et al. Mcl-1 is overexpressed in multiple myeloma and associated with relapse and shorter survival. Leukemia 2005; 19: 1248–1252.
Wei G, Twomey D, Lamb J, Schlis K, Agarwal J, Stam RW et al. Gene expression-based chemical genomics identifies rapamycin as a modulator of MCL1 and glucocorticoid resistance. Cancer Cell 2006; 10: 331–342.
Perciavalle RM, Stewart DP, Koss B, Lynch J, Milasta S, Bathina M et al. Anti-apoptotic MCL-1 localizes to the mitochondrial matrix and couples mitochondrial fusion to respiration. Nat Cell Biol 2012; 14: 575–583.
Velours J, Dautant A, Salin B, Sagot I, Brethes D . Mitochondrial F1F0-ATP synthase and organellar internal architecture. Int J Biochem Cell Biol 2009; 41: 1783–1789.
Gray MW, Burger G, Lang BF . The origin and early evolution of mitochondria. Genome Biol 2001; 2: : REVIEWS1018.
Galluzzi L, Joza N, Tasdemir E, Maiuri MC, Hengartner M, Abrams JM et al. No death without life: vital functions of apoptotic effectors. Cell Death Differ 2008; 15: 1113–1123.
Yip KW, Reed JC . Bcl-2 family proteins and cancer. Oncogene 2008; 27: 6398–6406.
Tsujimoto Y, Cossman J, Jaffe E, Croce CM . Involvement of the bcl-2 gene in human follicular lymphoma. Science 1985; 228: 1440–1443.
Acknowledgements
This work was funded by Health Research Board (RP/2008/14) and Science Foundation Ireland (08/IN1/1949) grants to JHMP.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Kilbride, S., Prehn, J. Central roles of apoptotic proteins in mitochondrial function. Oncogene 32, 2703–2711 (2013). https://doi.org/10.1038/onc.2012.348
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2012.348
Keywords
This article is cited by
-
SIRT6 regulates obesity-induced oxidative stress via ENDOG/SOD2 signaling in the heart
Cell Biology and Toxicology (2023)
-
Mitochondria dysfunction in CD8+ T cells as an important contributing factor for cancer development and a potential target for cancer treatment: a review
Journal of Experimental & Clinical Cancer Research (2022)
-
Duck Tembusu virus infection induces mitochondrial-mediated and death receptor-mediated apoptosis in duck embryo fibroblasts
Veterinary Research (2022)
-
Prevention of acquired sensorineural hearing loss in mice by in vivo Htra2 gene editing
Genome Biology (2021)
-
BH3-only protein expression determines hepatocellular carcinoma response to sorafenib-based treatment
Cell Death & Disease (2021)