Published online Mar 7, 2011. doi: 10.3748/wjg.v17.i9.1219
Revised: December 9, 2010
Accepted: December 16, 2010
Published online: March 7, 2011
AIM: To study the correlation between high metastasis-associated protein 1 (MTA1) expression and lymphangiogenesis in colorectal cancer (CRC) and its role in production of vascular endothelial growth factor-C(VEGF-C).
METHODS: Impact of high MTA1 and VEGF-C expression levels on disease progression and lymphovascular density (LVD, D2-40-immunolabeled) in 81 cases of human CRC was evaluated by immunohistochemistry. VEGF-C mRNA and protein expressions in human LoVo and HCT116 cell lines were detected by real-time polymerase chain reaction and Western blotting, respectively, with a stable expression vector or siRNA.
RESULTS: The elevated MTA1 and VEGF-C expression levels were correlated with lymph node metastasis and Dukes stages (P < 0.05). Additionally, high MTA1 expression level was correlated with a large tumor size (P < 0.05). A significant correlation was found between MTA1 and VEGF-C protein expressions in tumor cells (r = 0.371, P < 0.05). Similar to the VEGF-C expression level, high MTA1 expression level was correlated with high LVD in CRC (P < 0.05). Furthermore, over-expression of MTA1 significantly enhanced the VEGF-C mRNA and protein expression levels, whereas siRNAs - knocked down MTA1 decreased the VEGF-C expression level.
CONCLUSION: MTA1, as a regulator of tumor-associated lymphangiogenesis, promotes lymphangiogenesis in CRC by mediating the VEGF-C expression.
- Citation: Du B, Yang ZY, Zhong XY, Fang M, Yan YR, Qi GL, Pan YL, Zhou XL. Metastasis-associated protein 1 induces VEGF-C and facilitates lymphangiogenesis in colorectal cancer. World J Gastroenterol 2011; 17(9): 1219-1226
- URL: https://www.wjgnet.com/1007-9327/full/v17/i9/1219.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i9.1219
Lymphatic metastasis is one of the most important metastatic routes of epithelial cancer including colorectal cancer (CRC)[1]. Excessive formation of new lymphatics (lymphangiogenesis) in CRC is a key process for lymphatic metastasis, and lymphatic vessel density (LVD) is an indicator for lymphangiogenesis[1-3]. Metastasis-associated protein 1 (MTA1) is expressed in a wide range of epithelial cancers and plays a crucial role in tumor metastasis[4]. MTA1 has been established as the only single gene showing consistently increased in lymph node metastases of head and neck squamous cell carcinoma[5]. A large number of clinicopathological studies show that elevated MTA1 is correlated with lymph node metastasis of epithelial cancer[4,6-9]. These data indicate that MTA1 is involved in lymphatic metastasis of epithelial cancer. However, whether the pro-metastatic effects of MTA1 are mediated by promoting lymphangiogenesis remains unknown.
MTA1, as a part of the nucleosome remodeling and deacetylation complex (NuRD), promotes metastasis of cancer by regulating many tumor-associated genes[4,9]. For example, MTA1 physically binds to hypoxia-inducible factor -1α (HIF-1α) and increases its transcriptional activity, leading to enhanced expression of vascular endothelial growth factor (VEGF)-A which is associated with metastasis[10,11]. VEGF-C is another member of the VEGF family and the major regulatory factor for tumor lymphangiogenesis[1,2]. Similar to VEGF-A, VEGF-C is also directly induced by HIF-1α[12-14], and therefore, is a potential target molecule of MTA1. Thus, whether MTA1 can promote lymphangiogenesis by up-regulating the expression of VEGF-C in CRC is unknown and worthy of further study.
In this study, the expression of MTA1 was correlated with VEGF-C in CRC specimens. MTA1 was related with high LVD, indicating that MTA1 promotes lymphangiogenesis and thus induces metastasis of tumor. Furthermore, MTA1 mediated VEGF-C expression, suggesting MTA1 can promote lymphangiogenesis by increasing the VEGF-C expression level in CRC.
Eighty-one CRC tissue specimens, provided by Department of Pathology, First Affiliated Hospital of Jinan University, were fixed in 10% buffered formalin for 24-48 h,embedded in paraffin wax, and then cut into sections which were stained with hematoxylin and eosin and reviewed twice by two experienced pathologists to verify the diagnosis, histological grade and type, based on the system of the Union for International Cancer Control (UICC). A synopsis of the clinicopathological parameters is provided in Table 1.
| Parameters of patients | MTA1 | VEGF-C | |||||
| n | High | Low | P value | High | Low | P value | |
| Age | |||||||
| ≤ 60 | 34 | 11 | 23 | 0.805 | 16 | 18 | 0.552 |
| > 60 | 47 | 14 | 33 | 19 | 28 | ||
| Gender | |||||||
| Male | 45 | 17 | 28 | 0.132 | 21 | 24 | 0.483 |
| Female | 36 | 8 | 28 | 14 | 22 | ||
| Tumor Size | |||||||
| ≤ 5 cm | 46 | 10 | 36 | 0.042 | 18 | 28 | 0.395 |
| > 5 cm | 35 | 15 | 20 | 17 | 18 | ||
| Location | |||||||
| Colon | 47 | 16 | 31 | 0.467 | 18 | 29 | 0.294 |
| Rectum | 34 | 9 | 25 | 17 | 17 | ||
| Depth of invasion | |||||||
| T1 + T2 | 15 | 3 | 12 | 0.313 | 4 | 11 | 0.152 |
| T3 + T4 | 66 | 22 | 44 | 31 | 35 | ||
| Differentiation | |||||||
| Well, moderately | 59 | 15 | 44 | 0.083 | 24 | 35 | 0.451 |
| Poorly | 22 | 10 | 12 | 11 | 11 | ||
| Dukes stages | |||||||
| A + B | 41 | 8 | 33 | 0.004 | 10 | 31 | 0.001 |
| C + D | 40 | 17 | 23 | 25 | 15 | ||
| Lymph node metastasis | |||||||
| Yes | 40 | 17 | 23 | 0.025 | 24 | 16 | 0.003 |
| No | 41 | 8 | 33 | 11 | 30 | ||
The sections of CRC tissue specimen were stained with immunohistochemistry (IHC) using a streptavidin-peroxidase technique (Beijing Zhong Shan Golden Bridge Biological Technology Co., Ltd., China). Briefly, the sections were incubated in methanol/H2O2 for 30 min to inhibit the endogenous peroxidase activity, washed with PBS for 5 min and blocked with normal goat serum for 20 min at room temperature. The sections were incubated with antibodies against MTA1 (sc-9446; Santa Cruz Biotechnology Inc., CA, USA) or VEGF-C (sc-7133; Santa Cruz Biotechnology Inc., CA, USA) overnight at 4°C, then with biotinylated secondary antibody for 1 h at room temperature and avidin-conjugated peroxidase for 45 min at room temperature. The sections were washed three times with PBS between each step. Peroxidase was stained with diaminobenzidine (1 mg/mL) and H2O2 for 5 min and washed with tap water for 10 min. The sections were counterstained with hematoxylin for 1 min. PBS was used as a negative control instead of primary antibody.
Two investigators who were blinded to the patient outcomes and all clinicopathological findings examined the stainings independently. Positive expression of MTA1 and VEGF-C was detected by estimating the staining intensity and the percentage of tumor cells showing specific immunoreactivity. Samples with 10% tumor cells were defined as positive. The intensity score was defined as 0 (no staining), 1 (weak staining), 2 (moderate staining), and 3 (strong staining), respectively, as previously described[15,16]. Tumors with a score > 2 (moderate and strong expression) showed a high expression level of MTA1 and VEGF-C[15,16].
LVD in CRC tissue sections that were single-stained for D2-40 was quantitatively analyzed as previously described[3,17]. Tumoral LVD located at the periphery of tissue, within 2 mm of the tumor and adjacent to the invasive front was assessed. Five areas with most lymphatic regions (“hot spots”) were chosen by light microscopy at 40 × magnification. LVD was assessed by counting all stained vessels at 200 × magnification. The assessed mean number of lymphatics was determined and expressed as LVD[17,18]. The LVD was scored and counted by two investigators independently who were blinded to clinical information about the patients.
Human CRC cell lines, HCT116 and LoVo, purchased from Center of Experimental Animals, Sun Yat-Sen University (Guangzhou, China), were cultured in Dulbecco’s modified Eagle’s medium (Life Technologies Corporation, CA, USA) containing 100 IU/mL penicillin, 100 μg/mL streptomycin, and 10% heat-inactivated fetal bovine serum in a humidified atmosphere containing 5mL CO2 at 37°C.
Two 21-nucleotide MTA1 siRNAs (si-MTA1#1 and si-MTA1#2) were designed to target the sequences 5'-GGAGAAUCGAGGAGCUCAACA-3' and 5'-GCAUCAUUGAGUACUACUACA-3' of MTA1 mRNA (NCBI accession number: NM_004689.3). BLAST searches indicated that the targeted regions had no significant homology with any other genes. A non-targeting siRNA was used as a negative control (NC, 5'-UUCUCCGAACGUGUCACGUTT-3') in all siRNA transfection experiments. All siRNAs, purchased from Shanghai GenePharma Co., Ltd (Shanghai, China) were transfected twice at 24-h intervals with Oligofectamine reagent (Life Technologies Corporation, CA, USA) according to its manufacturer’s protocol[19].
Plasmid MTA1-EGFP-C1 (a gift from Professor Chen Anman at Huazhong University of Science and Technology, China)[20] and plasmid EGFP-C1 vector (as a blank control) was transfected into LoVo cells with Oligofectamine reagent (Life Technologies Corporation, CA, USA) according to its manufacturer’s protocol. Medium was supplemented with 400-800 μg /mL G418 (Life Technologies Corporation, CA, USA) to screen for positive cells, then with 400 μg/mL G418 after two weeks. Positive cell clones were isolated by ring chining and further expanded for genetic and functional characterization. Expression of MTA1 was detected by real-time polymerase chain reaction (PCR) and Western blotting, respectively.
MTA1 and VEGF-C mRNA was analyzed by real-time PCR as previously described[19]. Briefly, total RNA was extracted from cells using TRIzol (Life Technologies Corporation, CA, USA). First strand cDNA was synthesized from mRNA using a Primescript™ RT reagent kit (TaKaRa, Tokyo, Japan). Real-time PCR was carried out using the SYBR Premix ExTaq™ (TaKaRa, Tokyo, Japan) according to its manufacturer’s instructions. The input was normalized by GAPDH mRNA. Gene-specific primers are as follows: 5'-AGCTACGAGCAGCACAACGGGGT-3' (forward primer for mta1) and 5'- CACGCTTGGTTTCCGAGGAT-3' (reverse primer, 289 bp), 5'-AACCTCCATGTGTGTCCGTC-3' (forward primer for vegf-c) and 5'-TGGCAAAACTGATTGTTACTGG-3' (reverse primer, 156 bp), 5'-ACAGTCCATGCCATCACTGCC-3' (forward primer for GAPDH) and 5'-GCCTGCTTCACCACCTTCTTG-3' (reverse primer, 266 bp). Experiments were performed in triplicate, with the results represented as mean ± SE.
MTA1 and VEGF-C protein expression was detected by Western blotting as previously described[21]. Briefly, the cells were lysed in a RIPA buffer (1% NP-40, 150 mm NaCl, 0.05% DOC, 1% SDS, and 50 mM TrisCl, pH = 7.5) containing protease inhibitors. After separated by SDS-PAGE, the proteins were transferred to polyvinylidene difluoride membranes and subjected to immunoblotting with antibodies against MTA1 (Santa Cruz Biochemistry, CA, USA), VEGF-C (Santa Cruz Biochemistry, CA, USA) and tubulin (Sigma-Aldrich, Inc., MI, USA) at 4°C overnight. After washed, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies and visualized using the ECL chemiluminescence system (Thermo Fisher Scientific Inc., MA, USA).
Statistical analysis was carried out using the SPSS 13.0 for Windows. Pearson’s χ2 test was used to examine the relation between immunostaining scores of two different markers within different clinicopathological subgroups. Spearman’s rank-order correlation was used to test immunostaining scores of the significant association between MTA1 and VEGF-C. Student’s t-test was used to determine differences in two data sets, including the effect of LVD and siRNA treatment or gene transfection on gene expression. All tests were two-tailed and P <0.05 was considered statistically significant.
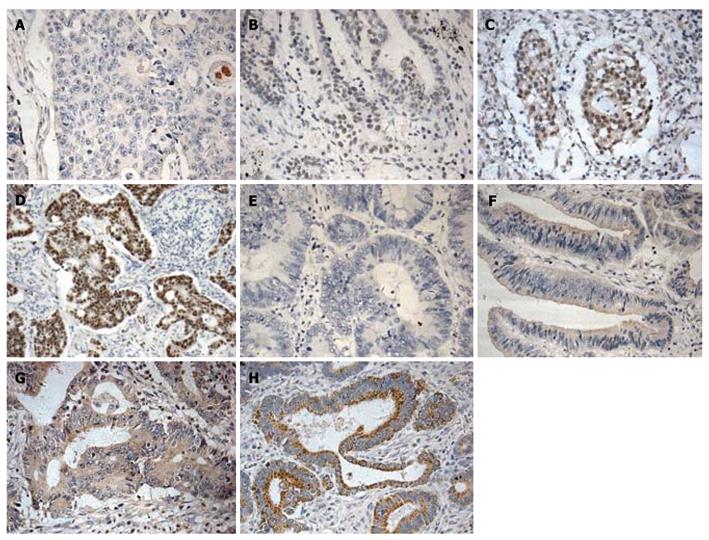
The expression of MTA1 and VEGF-C protein was detected in CRC tissue samples with IHC staining and the correlation between their expression and clinicopathological parameters was further analyzed. The MTA1 immunoreactivity was detected primarily in nuclei and weakly in cytoplasm of tumor cells with with IHC staining (Figure 1A-D), whereas positive VEGF-C staining was observed in cytoplasm (Figure 1E-H). However, faint staining of VEGF-C was occasionally observed in normal epithelial cells and stromal components showed, particularly in adjacent stromal endothelial cells, which is consistent with the reported findings[16,22,23].
The correlation between the MTA1 and VEGF-C expression and the clinicopathologic parameters is shown in Table 1. High MTA1 expression level was observed in 25 (30.8%) of the 81 tumor tissue samples, while high VEGF-C expression level was found in 35 (43.2%) of the 81 tumor tissue samples. The MTA1 and VEGF-C expressions were correlated with lymph node metastasis and Dukes stages (P < 0.05). Additionally, the high MTA1 expression level was correlated with a large tumor size (P < 0.05). Neither MTA1 nor VEGF-C was correlated with tumor location, differentiation, infiltration, and sex or age of the patients.
A significant correlation was found between MTA1 and VEGF-C protein expressions in tumor cells (r = 0.371, P < 0.05, Table 2), indicating that the high MTA1expression level can facilitate lymphatic metastasis of CRC.
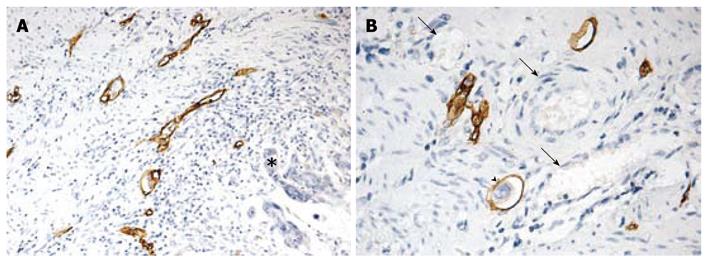
It has been reported that high peritumoral LVD is an independent risk factor for lymphangiogenesis[3,24]. Immunostaining of D2-40 is a specific marker for evaluation of lymphatic invasion and lymphatic microvessel density in human cancers[3,24,25]. To further determine whether MTA1 plays a role in lymphangiogenesis, the correlation between the expression of MTA1 and the quantity of D2-40 positive lymphatic vessels was analyzed in this study. The lymphatic vessels were lined with a single layer of D2-40 positive endothelial cells, while the adjacent blood vessels containing erythrocytes showed negative staining. Lymphatic vessels were distributed unevenly throughout the sections, and mostly located in the peritumoral area (Figure 2).
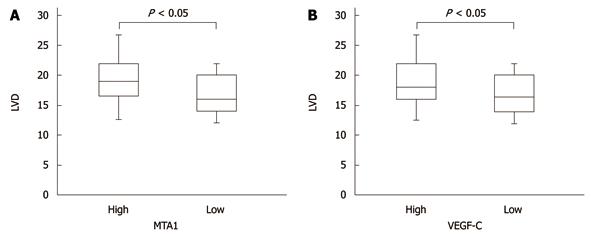
The LVD was 9- 31 with a mean of 17.76. The mean LVD was 19.92 and 19.60, respectively, in patients with a high MTA-1 and VEGF-C expression level, and 16.37 and 16.80, respectively, in those with a low, MTA-1 and VEGF-C expression level (P < 0.05), indicating that MTA1 also promotes lymphangiogenesis of CRC as VEGF-C. The correlation between MTA1 and VEGF-C expression and LVD is shown in Figure 3.
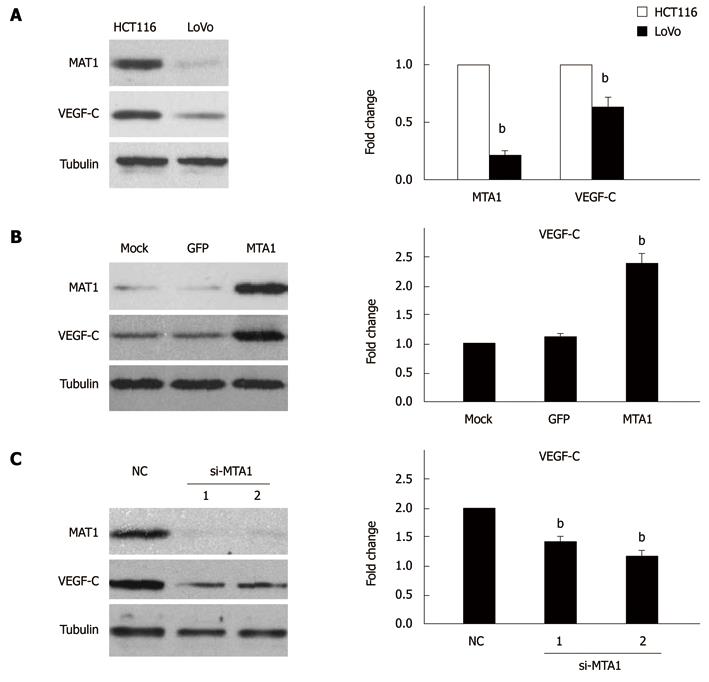
After a positive relation was established between MTA1 and VEGF-C expressions in CRC tissue specimens, whether MTA1 mediates VEGF-C expression in CRC cell lines was studied. HCT-116 cells with a high MTA1 level and LoVo cells with a low MTA1 level were used in our next experiments as previously described[26,27]. The expression of MTA1 and VEGF-C was detected in these two cell lines by real-time PCR and Western blotting, respectively. The MTA1 and VEGF-C mRNA and protein expression levels were significantly higher in HCT-116 cells than in LoVo cells (Figure 4A).
To determine whether MTA1 is responsible for the expression of VEGF-C, the MTA1 stable expression vector was used to mimic the MTA1 protein expression in LoVo cells, showing that the MTA1 stable expression vector significantly increased the MTA1 protein expression, while the VEGF-C protein expression level was remarkably up-regulated by the MTA1 stable expression vector compared to the mock and control vector (Figure 4B, left panel). Furthermore, over-expression of the MTA1 stable vector enhanced the VEGF-C mRNA expression level compared to the mock and control vector (Figure 4B, right panel), suggesting that MTA1 is required for VEGF-C expression.
To further specifically confirm the regulation of VEGF-C by MTA1, siRNA-mediated knockdown of MTA1 was employed. HCT116 cells were transfected with two siRNAs specific for MTA1. MTA1-targeted siRNAs specifically suppressed the MTA1 protein expression, while the level of an unrelated gene (like Tubulin) was unaffected, verifying that the siRNAs have a high selectivity and efficacy (Figure 4C, left panel). Furthermore, the silencing of MTA1 endogenous expression significantly decreased the VEGF-C protein expression, whereas the RNA interference technique had no significant effect on the VEGF-C expression as revealed by the negative control siRNA (NC), (Figure 4C, left panel), indicating that MTA1 is necessary for VEGF-C expression. Furthermore, knockdown of MTA1 expression decreased the VEGF-C mRNA expression level (Figure 4C, right panel), suggesting that MTA1 mediates VEGF-C expression in CRC cell lines.
This study investigated whether the expression of VEGF-C regulated by MTA1 affects the lymphangiogenesis of colon cancer. IHC studies in CRC specimens and mechanistic studies with genetic manipulation in colon cancer cell lines, demonstrated that MTA1-mediated expression of VEGF-C could facilitate the lymphangiogenesis of colon cancer.
MTA1 is associated with progression to the metastatic state in many epithelial cancers, including carcinomas of breast[6,28], prostate[22], and esophagus[7], as well as head and neck squamous cell carcinoma[5]. However, the relation between MTA1 and CRC metastasis is not clear. It has been showed that the expression of MTA1 mRNA in CRC is significantly correlated to the depth of invasion and lymph node metastasis[29]. It was reported that the expression level of MTA1 mRNA is higher in colorectal cancer tissue than in its adjacent normal tissue[30]. In this study, the MTA1 protein expression in CRC tissue specimens was evaluated with IHC staining, showing that the MTA1 expression level was significantly higher in primary CRC tumors with lymph node metastases than in those without lymph node metastases, which is consistent with the reported data[29,30].
Hematogenous and lymphatic metastases are the two main forms of tumor metastasis to distant organs. Jang et al[31] examined MTA1 protein expression and microvessel density (MVD) in breast cancer tissue specimens and showed that MTA1 overexpression is significantly correlated with high MVD. The proangiogenic effects of MTA1 have also been observed in hepatocellular carcinoma[32] and prostate cancer[22]. In this study, the over-expression of MTA1 was significantly associated with the increased tumor LVD, indicating that MTA1 facilitates tumor metastasis by up-regulating angiogenesis and lymphangiogenesis.
The presence of VEGF-C, an independent predictor of poor survival, is associated with lymphangiogenesis[17,23]. In this study, the expression of VEGF-C was correlated with lymphatic metastases and greater LVD in CRC, which is consistent with the reported findings[1,23,27]. More importantly, the lymphangiogenesis up-regulated by MTA1 was related to the induction of VEGF-C, the expression of MTA1 in CRC tissue specimens was associated with that of VEGF-C, over-expression of MTA1 increased the expression level of VEGF-C, and knockdown of MTA1 reduced it in CRC cell lines, indicating that MTA1 mediates the induction of VEGF-C during lymphangiogenesis of CRC.
Although we demonstrated MTA1 could mediate VEGF-C expression, its precise mechanism remains to be defined. MTA1 regulates the expression of target genes by inhibiting or promoting their transcriptional activity. For example, MTA1 has a strong transcription repressing activity on estrogen receptor-α[33] and BRCA1[34] (breast cancer type 1 susceptibility protein) by forming a complex with NuRD. However, other studies demonstrate that MTA1 stimulates the transcriptional activity of several gene promoters by interacting with RNA polymerase II[35,36]. Moreover, the expression of MTA1 can be strongly induced under hypoxic conditions in breast cancer cell lines, and MTA1 over-expression increases the transcriptional activity and stability of HIF-1α protein[10,11]. Hypoxia is one of the most powerful inducers of lymphangiogenesis, and VEGF-C is a target molecule of HIF-1α[13,14,37,38]. Therefore, MTA1 can promote VEGF-C expression through the HIF-1α pathways. The precise mechanism involved in MTA1 targeting of VEGF-C should be further studied.
In conclusion, MTA1 plays a role in lymphangiogenesis of human CRC by up-regulating VEGF-C, a key regulator of lymphangiogenic factors, thus providing a novel mechanism underlying the relation between the high MTA1 level, cancer metastasis and poor outcome. Therefore, MTA1 is a potentially novel target for the treatment of lymphatic metastasis of human CRC.
Excessive formation of new lymphatics (lymphangiogenesis) in colorectal cancer (CRC) facilitates metastasis of cancer cells to lymph nodes and distant organs. Metastasis-associated protein 1 (MTA1) is expressed in a wide range of epithelial cancers including CRC, and plays a crucial role in their metastasis. Vascular endothelial growth factor-C (VEGF-C) is the major regulatory factor for CRC lymphangiogenesis.
MTA1 is involved in regulation of VEGF family during metastasis. Whether MTA1 promotes lymphangiogenesis by up-regulating the expression of VEGF-C in CRC needs to be further studied.
This is the first study to demonstrate that MTA1 plays a role in lymphangiogenesis by up-regulating VEGF-C, thus providing a novel mechanism of MTA1 underlying the promotion of CRC metastasis.
The findings in this study are of value in explanation of the mechanism of CRC lymphangiogenesis. MTA1 may be used as a new potential target for the treatment of lymphatic metastasis of human CRC, although further animal experiments are required to confirm its activity in promoting lymphangiogenesis of CRC.
It is a well written manuscript with promising results that may be the basis of forthcoming new research in CRC biology and therapy. The correlation between MTA1 and VEGF-C is of great clinical importance, as new therapeutic targets may be identified based on the results of this study.
Peer reviewer: Ferenc Sipos, MD, PhD, Cell Analysis Laboratory, 2nd Department of Internal Medicine, Semmelweis University, Szentkirályi u. 46., Budapest 1088, Hungary
S- Editor Sun H L- Editor Wang XL E- Editor Ma WH
| 1. | Royston D, Jackson DG. Mechanisms of lymphatic metastasis in human colorectal adenocarcinoma. J Pathol. 2009;217:608-619. [Cited in This Article: ] |
| 2. | Achen MG, Stacker SA. Molecular control of lymphatic metastasis. Ann N Y Acad Sci. 2008;1131:225-234. [Cited in This Article: ] |
| 3. | Lin M, Ma SP, Lin HZ, Ji P, Xie D, Yu JX. Intratumoral as well as peritumoral lymphatic vessel invasion correlates with lymph node metastasis and unfavourable outcome in colorectal cancer. Clin Exp Metastasis. 2010;27:123-132. [Cited in This Article: ] |
| 4. | Toh Y, Nicolson GL. The role of the MTA family and their encoded proteins in human cancers: molecular functions and clinical implications. Clin Exp Metastasis. 2009;26:215-227. [Cited in This Article: ] |
| 5. | Roepman P, de Jager A, Groot Koerkamp MJ, Kummer JA, Slootweg PJ, Holstege FC. Maintenance of head and neck tumor gene expression profiles upon lymph node metastasis. Cancer Res. 2006;66:11110-11114. [Cited in This Article: ] |
| 6. | Martin MD, Fischbach K, Osborne CK, Mohsin SK, Allred DC, O'Connell P. Loss of heterozygosity events impeding breast cancer metastasis contain the MTA1 gene. Cancer Res. 2001;61:3578-3580. [Cited in This Article: ] |
| 7. | Toh Y, Ohga T, Endo K, Adachi E, Kusumoto H, Haraguchi M, Okamura T, Nicolson GL. Expression of the metastasis-associated MTA1 protein and its relationship to deacetylation of the histone H4 in esophageal squamous cell carcinomas. Int J Cancer. 2004;110:362-367. [Cited in This Article: ] |
| 8. | Yi S, Guangqi H, Guoli H. The association of the expression of MTA1, nm23H1 with the invasion, metastasis of ovarian carcinoma. Chin Med Sci J. 2003;18:87-92. [Cited in This Article: ] |
| 9. | Nicolson GL, Nawa A, Toh Y, Taniguchi S, Nishimori K, Moustafa A. Tumor metastasis-associated human MTA1 gene and its MTA1 protein product: role in epithelial cancer cell invasion, proliferation and nuclear regulation. Clin Exp Metastasis. 2003;20:19-24. [Cited in This Article: ] |
| 10. | Yoo YG, Kong G, Lee MO. Metastasis-associated protein 1 enhances stability of hypoxia-inducible factor-1alpha protein by recruiting histone deacetylase 1. EMBO J. 2006;25:1231-1241. [Cited in This Article: ] |
| 11. | Moon HE, Cheon H, Chun KH, Lee SK, Kim YS, Jung BK, Park JA, Kim SH, Jeong JW, Lee MS. Metastasis-associated protein 1 enhances angiogenesis by stabilization of HIF-1alpha. Oncol Rep. 2006;16:929-935. [Cited in This Article: ] |
| 12. | Nilsson I, Shibuya M, Wennström S. Differential activation of vascular genes by hypoxia in primary endothelial cells. Exp Cell Res. 2004;299:476-485. [Cited in This Article: ] |
| 13. | Simiantonaki N, Jayasinghe C, Michel-Schmidt R, Peters K, Hermanns MI, Kirkpatrick CJ. Hypoxia-induced epithelial VEGF-C/VEGFR-3 upregulation in carcinoma cell lines. Int J Oncol. 2008;32:585-592. [Cited in This Article: ] |
| 14. | Mikhaylova M, Mori N, Wildes FB, Walczak P, Gimi B, Bhujwalla ZM. Hypoxia increases breast cancer cell-induced lymphatic endothelial cell migration. Neoplasia. 2008;10:380-389. [Cited in This Article: ] |
| 15. | Hofer MD, Kuefer R, Varambally S, Li H, Ma J, Shapiro GI, Gschwend JE, Hautmann RE, Sanda MG, Giehl K. The role of metastasis-associated protein 1 in prostate cancer progression. Cancer Res. 2004;64:825-829. [Cited in This Article: ] |
| 16. | Miyake K, Yoshizumi T, Imura S, Sugimoto K, Batmunkh E, Kanemura H, Morine Y, Shimada M. Expression of hypoxia-inducible factor-1alpha, histone deacetylase 1, and metastasis-associated protein 1 in pancreatic carcinoma: correlation with poor prognosis with possible regulation. Pancreas. 2008;36:e1-e9. [Cited in This Article: ] |
| 17. | Aishima S, Nishihara Y, Iguchi T, Taguchi K, Taketomi A, Maehara Y, Tsuneyoshi M. Lymphatic spread is related to VEGF-C expression and D2-40-positive myofibroblasts in intrahepatic cholangiocarcinoma. Mod Pathol. 2008;21:256-264. [Cited in This Article: ] |
| 18. | Lahat G, Lazar A, Wang X, Wang WL, Zhu QS, Hunt KK, Pollock RE, Lev D. Increased vascular endothelial growth factor-C expression is insufficient to induce lymphatic metastasis in human soft-tissue sarcomas. Clin Cancer Res. 2009;15:2637-2646. [Cited in This Article: ] |
| 19. | Du B, Ma LM, Huang MB, Zhou H, Huang HL, Shao P, Chen YQ, Qu LH. High glucose down-regulates miR-29a to increase collagen IV production in HK-2 cells. FEBS Lett. 2010;584:811-816. [Cited in This Article: ] |
| 20. | Yi C, Li X, Xu W, Chen A. Relationship between the expression of MTA-1 gene and the metastasis and invasion in human osteosarcoma. J Huazhong Univ Sci Technolog Med Sci. 2005;25:445-447. [Cited in This Article: ] |
| 21. | Du B, Zhong X, Liao X, Xu W, Zhou X, Xu S. A new antitumor arabinopyranoside from Laurencia majuscula induces G2/M cell cycle arrest. Phytother Res. 2010;24:1447-1450. [Cited in This Article: ] |
| 22. | Kai L, Wang J, Ivanovic M, Chung YT, Laskin WB, Schulze-Hoepfner F, Mirochnik Y, Satcher RL Jr, Levenson AS. Targeting prostate cancer angiogenesis through metastasis-associated protein 1 (MTA1). Prostate. 2011;71:268-280. [Cited in This Article: ] |
| 23. | Akagi K, Ikeda Y, Miyazaki M, Abe T, Kinoshita J, Maehara Y, Sugimachi K. Vascular endothelial growth factor-C (VEGF-C) expression in human colorectal cancer tissues. Br J Cancer. 2000;83:887-891. [Cited in This Article: ] |
| 24. | Barresi V, Reggiani-Bonetti L, Di Gregorio C, De Leon MP, Barresi G. Lymphatic vessel density and its prognostic value in stage I colorectal carcinoma. J Clin Pathol. 2011;64:6-12. [Cited in This Article: ] |
| 25. | Yonemura Y, Endou Y, Tabachi K, Kawamura T, Yun HY, Kameya T, Hayashi I, Bandou E, Sasaki T, Miura M. Evaluation of lymphatic invasion in primary gastric cancer by a new monoclonal antibody, D2-40. Hum Pathol. 2006;37:1193-1199. [Cited in This Article: ] |
| 26. | Cimen I, Tunçay S, Banerjee S. 15-Lipoxygenase-1 expression suppresses the invasive properties of colorectal carcinoma cell lines HCT-116 and HT-29. Cancer Sci. 2009;100:2283-2291. [Cited in This Article: ] |
| 27. | Zhong D, Li Y, Peng Q, Zhou J, Zhou Q, Zhang R, Liang H. Expression of Tiam1 and VEGF-C correlates with lymphangiogenesis in human colorectal carcinoma. Cancer Biol Ther. 2009;8:689-695. [Cited in This Article: ] |
| 28. | Martin MD, Hilsenbeck SG, Mohsin SK, Hopp TA, Clark GM, Osborne CK, Allred DC, O'Connell P. Breast tumors that overexpress nuclear metastasis-associated 1 (MTA1) protein have high recurrence risks but enhanced responses to systemic therapies. Breast Cancer Res Treat. 2006;95:7-12. [Cited in This Article: ] |
| 29. | Toh Y, Oki E, Oda S, Tokunaga E, Ohno S, Maehara Y, Nicolson GL, Sugimachi K. Overexpression of the MTA1 gene in gastrointestinal carcinomas: correlation with invasion and metastasis. Int J Cancer. 1997;74:459-463. [Cited in This Article: ] |
| 30. | Giannini R, Cavallini A. Expression analysis of a subset of coregulators and three nuclear receptors in human colorectal carcinoma. Anticancer Res. 2005;25:4287-4292. [Cited in This Article: ] |
| 31. | Jang KS, Paik SS, Chung H, Oh YH, Kong G. MTA1 overexpression correlates significantly with tumor grade and angiogenesis in human breast cancers. Cancer Sci. 2006;97:374-379. [Cited in This Article: ] |
| 32. | Ryu SH, Chung YH, Lee H, Kim JA, Shin HD, Min HJ, Seo DD, Jang MK, Yu E, Kim KW. Metastatic tumor antigen 1 is closely associated with frequent postoperative recurrence and poor survival in patients with hepatocellular carcinoma. Hepatology. 2008;47:929-936. [Cited in This Article: ] |
| 33. | Talukder AH, Mishra SK, Mandal M, Balasenthil S, Mehta S, Sahin AA, Barnes CJ, Kumar R. MTA1 interacts with MAT1, a cyclin-dependent kinase-activating kinase complex ring finger factor, and regulates estrogen receptor transactivation functions. J Biol Chem. 2003;278:11676-11685. [Cited in This Article: ] |
| 34. | Molli PR, Singh RR, Lee SW, Kumar R. MTA1-mediated transcriptional repression of BRCA1 tumor suppressor gene. Oncogene. 2008;27:1971-1980. [Cited in This Article: ] |
| 35. | Gururaj AE, Singh RR, Rayala SK, Holm C, den Hollander P, Zhang H, Balasenthil S, Talukder AH, Landberg G, Kumar R. MTA1, a transcriptional activator of breast cancer amplified sequence 3. Proc Natl Acad Sci USA. 2006;103:6670-6675. [Cited in This Article: ] |
| 36. | Balasenthil S, Gururaj AE, Talukder AH, Bagheri-Yarmand R, Arrington T, Haas BJ, Braisted JC, Kim I, Lee NH, Kumar R. Identification of Pax5 as a target of MTA1 in B-cell lymphomas. Cancer Res. 2007;67:7132-7138. [Cited in This Article: ] |
| 37. | Liang X, Yang D, Hu J, Hao X, Gao J, Mao Z. Hypoxia inducible factor-alpha expression correlates with vascular endothelial growth factor-C expression and lymphangiogenesis/angiogenesis in oral squamous cell carcinoma. Anticancer Res. 2008;28:1659-1666. [Cited in This Article: ] |
| 38. | Spinella F, Garrafa E, Di Castro V, Rosanò L, Nicotra MR, Caruso A, Natali PG, Bagnato A. Endothelin-1 stimulates lymphatic endothelial cells and lymphatic vessels to grow and invade. Cancer Res. 2009;69:2669-2676. [Cited in This Article: ] |