Published online Oct 10, 2014. doi: 10.5306/wjco.v5.i4.677
Revised: March 7, 2014
Accepted: March 13, 2014
Published online: October 10, 2014
Cohesive scientific evidence from molecular, animal, and human investigations supports the hypothesis that constitutive overexpression of cyclooxygenase-2 (COX-2) is a ubiquitous driver of mammary carcinogenesis, and reciprocally, that COX-2 blockade has strong potential for breast cancer prevention and therapy. Key findings include the following: (1) COX-2 is constitutively expressed throughout breast cancer development and expression intensifies with stage at detection, cancer progression and metastasis; (2) essential features of mammary carcinogenesis (mutagenesis, mitogenesis, angiogenesis, reduced apoptosis, metastasis and immunosuppression) are linked to COX-2-driven prostaglandin E2 (PGE-2) biosynthesis; (3) upregulation of COX-2 and PGE-2 expression induces transcription of CYP-19 and aromatase-catalyzed estrogen biosynthesis which stimulates unbridled mitogenesis; (4) extrahepatic CYP-1B1 in mammary adipose tissue converts paracrine estrogen to carcinogenic quinones with mutagenic impact; and (5) agents that inhibit COX-2 reduce the risk of breast cancer in women without disease and reduce recurrence risk and mortality in women with breast cancer. Recent sharp increases in global breast cancer incidence and mortality are likely driven by chronic inflammation of mammary adipose and upregulation of COX-2 associated with the obesity pandemic. The totality of evidence clearly supports the supposition that mammary carcinogenesis often evolves as a progressive series of highly specific cellular and molecular changes in response to induction of constitutive over-expression of COX-2 and the prostaglandin cascade in the “inflammogenesis of breast cancer”.
Core tip: Mammary carcinogenesis often evolves as a series of highly specific cellular and molecular changes in response to induction of constitutive over-expression of cyclooxygenase-2 (COX-2) and the prostaglandin cascade; reciprocally, agents that block COX-2 have significant value in the chemoprevention and therapy of breast cancer.
- Citation: Harris RE, Casto BC, Harris ZM. Cyclooxygenase-2 and the inflammogenesis of breast cancer. World J Clin Oncol 2014; 5(4): 677-692
- URL: https://www.wjgnet.com/2218-4333/full/v5/i4/677.htm
- DOI: https://dx.doi.org/10.5306/wjco.v5.i4.677
More than a century ago, Virchow et al[1,2] suggested that chronic inflammation leads to cancer development by increasing cellular proliferation[3]. Various models of carcinogenesis have been proposed involving inflammatory stimuli and mediators of wound healing[4-6]. The recent discovery of the inducible cyclooxygenase-2 (COX-2) gene has rekindled interest in the causal link between inflammation and cancer, and various models of carcinogenesis have been proposed involving inflammatory stimuli and COX-2 expression[7-10].
The current review synthesizes and interprets the accumulating body of evidence supporting COX-2 driven inflammogenesis as a general model of breast cancer development and the use of anti-inflammatory compounds that block COX-2 for breast cancer prevention and therapy. Evidence from molecular studies and meta-analyses of COX-2-inhibiting agents and breast cancer are discussed and based upon results, a general model of inflammogenesis of breast cancer is proposed involving induction of constitutive COX-2 over-expression and up-regulation of the prostaglandin cascade.
Vane et al[11] discovered that the anti-inflammatory effects of aspirin [and all other nonsteroidal anti-inflammatory drugs (NSAIDs)] are primarily due to their inhibition of cyclooxygenase, the rate-limiting enzyme of the prostaglandin cascade. Metabolism of the essential fatty acid, arachidonic acid, via the cyclooxygenase pathway produces various prostaglandins that have a diverse array of physiologic activities throughout the human system. Indeed, these short lived molecules appear to control not only the inflammatory response, but they also help regulate constriction of blood vessels, contraction of smooth muscle, aggregation of platelets, sensitization of neurons to pain, flux of intracellular calcium, cell division, apoptosis, and many other molecular events that are critical for homeostatic physiology.
Two primary genes encode cyclooxygenase, a constitutive gene (COX-1) and its inducible isoform (COX-2)[12-14]. The inducible COX-2 gene is the master switch that activates the inflammatory response. Induction of COX-2 by any inflammatory stimulus (e.g., tobacco, alcohol, ischemia, trauma, pressure, foreign bodies, toxins, bacteria, viruses, lipopolysaccharides, etc.) quickly results in the biosynthesis of prostaglandins of the E-series, particularly prostaglandin E2 (PGE-2), and these prostaglandins in turn orchestrate the inflammatory response.
The cyclooxygenase pathway produces various prostaglandins, prostacyclins and thromboxanes from arachidonic acid and other fatty acids. In the initial step, COX catalyzes the oxidation of arachidonic acid to prostaglandin H-2 (PGH-2) which is rapidly converted to biologically active prostaglandins by specific enzymes. For example, PGH-2 is converted to the chief inflammatory prostaglandin, PGE-2, by PGE-2 synthetase.
Prostaglandin structure and function depend upon the cell of origin and the level and type of catalytic COX enzyme. COX-1 is constitutively expressed at basal levels in many cells throughout the body, e.g., gastrointestinal epithelium, renal tubules, vascular smooth muscle and blood platelets. Ordinarily, COX-1 expression is constitutive and sustains low levels of prostaglandins that are cytoprotective and maintain homeostasis. Conversely, the COX-2 gene is silent (not transcribed) unless induced by inflammatory stimuli. Induced COX-2 transcription and expression markedly amplify the biosynthesis of PGE-2 which is the chief effector molecule of inflammation[15].
Under normal conditions, acute inflammation is a tightly controlled self-limiting response to the offending stimulus. The process involves the integration of multiple cell types of the vascular and immune systems for the purpose of targeting, capturing, degrading, and removing the offending agent from the tissue under attack. Concurrent with acute inflammation, COX-2 expression and PGE-2 production by endothelial cells, epithelial cells, stromal cells, monocytes and lymphocytes increases up to 100 fold of basal levels. Amplification of the COX-2 inflammatory cascade is triggered by recognition of pro-inflammatory stimuli by toll-like receptors on the cell membranes of exposed cells and activation of nuclear factor kappa β (NF-κβ) which is often touted as a universal transcription factor[16]. In addition, a variety of cytokines are secreted by infiltrating macrophages and other cells of the innate immune system. In particular, tissue necrosis factor α, γ-interferon and interleukins 1 and 6 (IL-1 and IL-6), stimulate the production of acute phase proteins such as C-Reactive protein, Amyloid A and complement, which assist in the inflammatory response[17].
With abatement of the inflammatory stimulus, specific cytokines, particularly IL-1 and IL-6, exert feedback inhibition causing COX-2 expression and PGE-2 production to cease and the inflammatory process to subside. However, with sustained exposure to pro-inflammatory stimuli, continued overexpression of the COX-2 inflammatory cascade promotes the transition from acute to chronic inflammation. Molecular studies suggest that specific cytokines such as IL-6 and IL-1β are responsible for recruiting monocytes to chronically inflamed tissues which may in turn disrupt the inhibitory feedback loop by secreting a variety of other pro-inflammatory cytokines[18,19].
Constitutive expression of the COX-2 gene and sustained biosynthesis of PGE-2 appear to be irrevocably linked to the initiation and promotion of mammary carcinogenesis. This review builds upon the evidence from molecular studies of COX-2, the rate-limiting enzyme of the prostaglandin cascade, reflecting its virtually ubiquitous role in mammary carcinogenesis, and reciprocally, epidemiologic studies documenting the beneficial impact of COX-2 blockade in breast cancer prevention and therapy.
Molecular studies are reviewed and updated data compiled to elucidate the role of COX-2 in the progression of breast cancer[10]. Epidemiologic studies are reviewed and composite estimates derived by meta-analysis to quantify the impact of selective and non-selective agents that reduce breast cancer risk by inhibition of COX-2[20]. Convincing evidence is presented showing that mammary carcinogenesis often evolves as a progressive series of highly specific cellular and molecular changes in response to induction of constitutive over-expression of COX-2 and the prostaglandin cascade in the “inflammogenesis of breast cancer”.
Molecular studies using immunohistochemistry and reverse transcriptase polymerase chain reactions (RT-PCR) reveal that over-expression of COX-2 is a prominent feature of all stages of breast cancer. Furthermore, COX-2 is commonly found in premalignant lesions (dysplasia and atypia), carcinoma in situ, invasive cancer, and in particular, metastatic disease. In stark contrast to mammary cell populations that are in various stages of carcinogenesis, COX-2 is ordinarily not detectable in normal (non-inflamed) mammary tissues[21,22].
The first investigation of COX-2 in human breast cancer specimens was conducted using immunohistochemistry and a human COX-2 primer[23]. The study revealed the presence of COX-2 protein in 13 of 13 invasive human breast tumors, but not in samples of normal breast tissue. There was a statistically significant linear association between COX-2 and high (> 50%) tumor cell density (P < 0.01) with COX-2 protein localized to tumor cells.
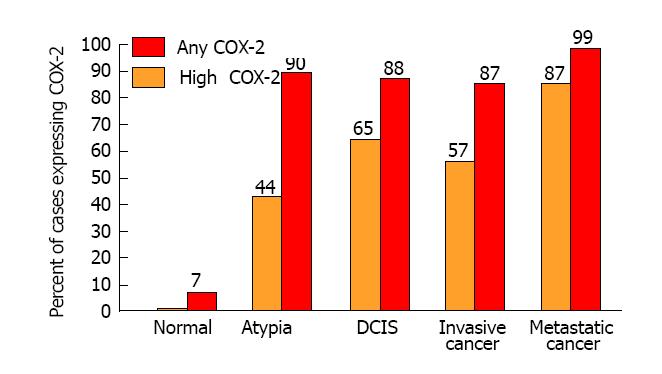
Subsequently, molecular biologists from multiple independent laboratories have consistently observed COX-2 over-expression in all stages of breast cancer[23-42]. Figure 1 shows the mean frequency of specimens over-expressing COX-2 in the progression of mammary carcinogenesis. Among studies of invasive breast cancer, 87% of specimens were positive for COX-2 and 57% had high levels of COX-2 expression. Significantly elevated frequencies of specimens with high COX-2 expression were also observed in premalignant lesions such as atypical hyperplasia (44%) and ductal carcinoma in situ (65%). Furthermore, several of the studies suggest that COX-2 expression is correlated with the metastatic spread of breast cancer and has strong potential as a prognostic indicator of disease severity and progression[30,31,35,37-39]. By comparison, all studies have found negligible or very weak focal COX-2 expression in normal tissues. It is indeed remarkable that high levels of COX-2 expression are evident throughout mammary carcinogenesis.
In an important prospective study conducted by Hartmann et al[43] at the Mayo Clinic, COX-2 expression was measured by immunohistochemistry in biopsy specimens from 235 women with atypical hyperplasia of the breast. Forty-one (17%) of the 235 women subsequently developed breast cancer during a median follow-up of 15 years. Notably, COX-2 expression at baseline was a significant predictor of risk. Compared to women without atypia, the cumulative incidence of breast cancer increased with increasing COX-2 expression, relative risk (RR) = 2.6 for weak or negligible expression, RR = 3.6 for moderate expression and RR = 5.7 for strong expression. The authors concluded that “COX-2 appears to be a biomarker that further stratifies breast cancer risk among women with atypia and may be a relevant target for chemoprevention strategies”[43,44].
The molecular evidence clearly demonstrates that COX-2 over-expression is not only an early event in the genesis of breast cancer, but is present throughout the entire evolutionary process of breast cancer development and progression. Thus, COX-2 may be a useful biomarker of impending cancer and a prime target for molecular intervention in breast cancer prevention and therapy[45].
The molecular evidence suggests that induction and constitutive upregulation of COX-2 and the prostaglandin cascade play a significant role in mammary carcinogenesis. But if inflammogenesis of breast cancer is to be upheld as a viable model, then the reciprocal relationship must also be true, vis a vis., blockade of COX-2 should have significant inhibitory impact against mammary carcinogenesis. Critical evidence from animal and human investigations is discussed next.
In the past quarter century, several independent investigations employing animal models of mammary carcinogenesis have generated compelling evidence that NSAIDs have significant and consistent chemopreventive effects against breast cancer development.
Karmali et al[46,47] first observed chemopreventive effects of NSAIDs against breast cancer and also elucidated differential effects of essential dietary fatty acids in prostaglandin (PG) biosynthesis and tumor promotion. Their studies showed that dietary supplementation with the n-6 fatty acid, linoleic acid, promoted tumor growth and development via enhanced arachidonic acid metabolism and elevated levels of PG activity, whereas the n-3 essential fatty acid, linolenic acid, had the opposite effect.
In several subsequent preclinical investigations of chemically induced breast cancer, supplemental administration of general NSAIDs such as aspirin, ibuprofen, piroxicam, sulindac, and others, in the diet or drinking water consistently reduced the growth and progression of breast tumors[48-50]. The molecular basis for the anti-neoplastic effects of these general NSAIDS is linked to their inhibition of cyclooxgenase gene expression and enzyme activity. However, general NSAIDs have nonselective activity against both COX-1 and COX-2.
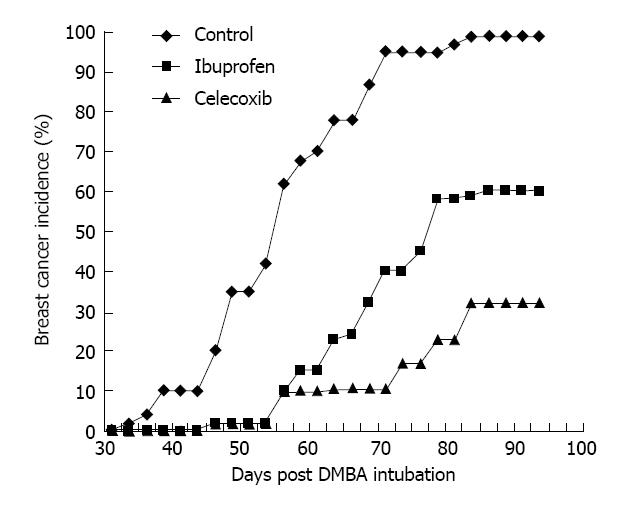
It is therefore important to note that recent preclinical studies have demonstrated even stronger antineoplastic effects of selective COX-2 inhibitors such as celecoxib, rofecoxib, valdecoxib, and nimesulide against breast cancer. Harris and colleagues initially reported that celecoxib markedly reduced the incidence of DMBA-induced breast cancer in Sprague-Dawley female rats[51]. In their study, celecoxib reduced the incidence of breast cancer by 70% compared to controls. In the same trial, ibuprofen reduced the incidence of breast cancer by 40% (Figure 2). Further evidence for the primary role of COX-2 in mammary carcinogenesis comes from transgenic mouse models in which the overexpression of COX-2 is sufficient to induce malignant transformation of normal epithelial cells of the mammary gland[52].
In summary, animal models of carcinogenesis provide compelling evidence that NSAIDs inhibit growth and development of breast tumors. While preclinical investigations provide consistent evidence that both selective and nonselective NSAIDs inhibit chemically induced carcinogenesis of mammary epithelial tumors, the strongest antineoplastic effects are clearly the result of intervention by administration of COX-2 blocking agents.
Meta-analysis of NSAIDs and breast cancer: Independent estimates from 37 studies were used in an updated meta-analysis of over-the-counter NSAIDs (primarily aspirin or ibuprofen) and breast cancer[53-91]. These reports were ascertained by a search of MEDLINE in the period 1970-2013 using combinations of key words: breast cancer with NSAIDs, aspirin and ibuprofen. Methods developed by Schlesselman and Greenland were adapted for combined analysis of the data from these studies[92,93]. Estimates of RR and 95% confidence intervals were converted to ln(RR) with corresponding variance estimates (v). The combined estimate of risk in logarithmic form, ln(RR*)=∑ln(RR)w/∑w, was obtained by weighting individual estimates by w=1/v. A χ2 test of heterogeneity was utilized to test for differences among studies.
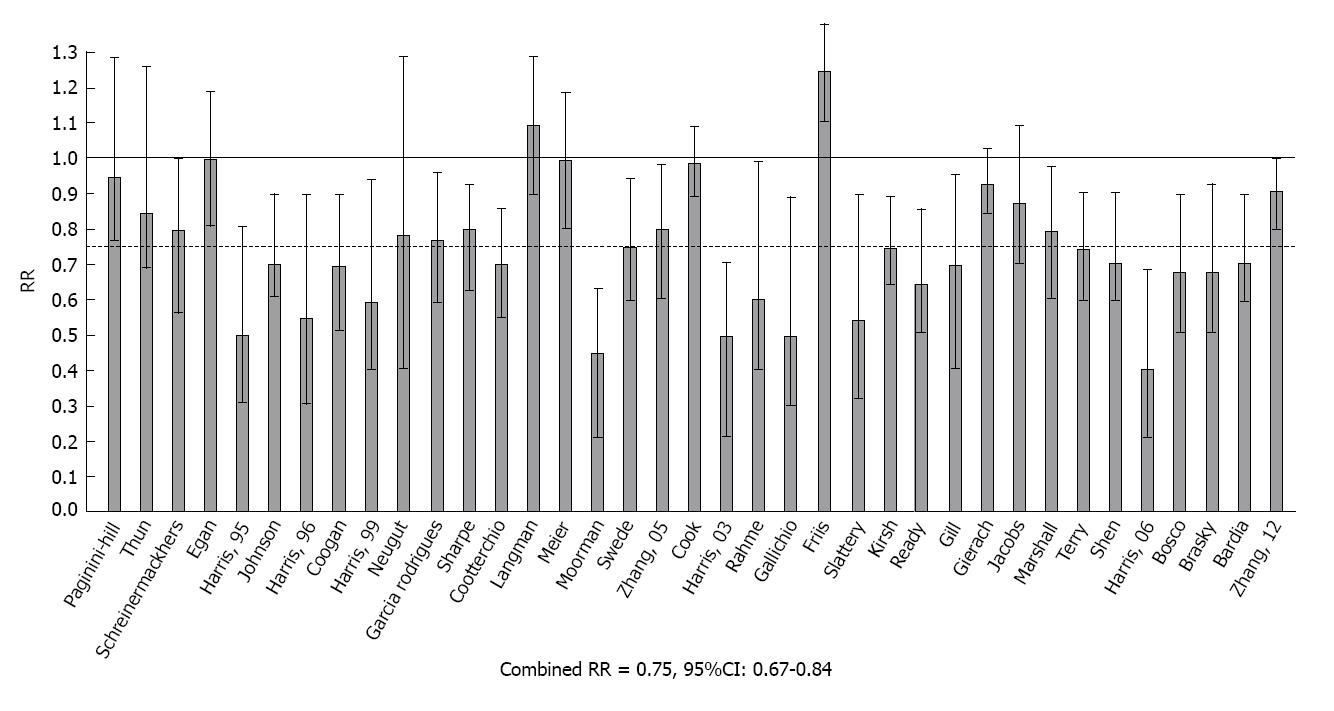
RR with 95%CIs from these reports are shown in Figure 3. Among the 37 estimates, 25 were significantly less than 1.0 and only one was significantly greater than 1.0. The test for heterogeneity was not significant and the composite estimate shows a 25% reduction in the relative risk of breast cancer with regular use of aspirin or other OTC NSAIDs (Combined RR = 0.75, 95%CI: 0.67-0.84, P < 0.001).
Our review and meta-analysis of data from the epidemiologic literature therefore provides compelling evidence that regular intake of NSAIDs that nonselectively block COX-2 protects against the development of breast cancer. When data are combined by meta-analysis, it is estimated that regular NSAID intake is associated with a 25% reduction in overall breast cancer risk. This estimate is similar to the results of earlier meta-analysis by González-Pérez et al[94] who reported a 23% reduction in breast cancer risk with NSAID use[93]. The available data suggest that significant reductions in breast cancer risk occur with 5 or more years of using low dosages of aspirin or other NSAIDs on a regular basis and long term studies suggest that the risk declines to maximal levels with regular intake for 10-20 years[94]. It is also notable that some studies have found that NSAIDs may have a greater effect against estrogen receptor positive breast cancer[72,77,80,86], and in one such study, a genetic polymorphism of the COX-2 gene was associated with a significant reduction in the risk of estrogen positive breast cancer[80].
Based on the epidemiologic evidence that nonselective NSAIDs reduce human breast cancer risk, we initiated a case control study of selective COX-2 inhibitors to assess their effects on the relative risk of breast cancer. The study was conducted for women diagnosed with breast cancer during the window of time (1998-2004) in which two selective COX-2 inhibitors, celecoxib and rofecoxib, were available by prescription in the United States. In the study, 323 cases with pathologically confirmed invasive breast cancer were compared to 649 controls without cancer who were frequency-matched at a 2:1 rate to the cases by age and county of residence[79].
Results of the investigation are shown in Table 1. Coxib use reduced the risk of breast cancer development by 71% (OR = 0.29, P < 0.01). Significant reductions in breast cancer risk were also noted for ibuprofen (63%) and regular 325 mg aspirin (49%) but not for low dose (81 mg) aspirin (23%). There was no effect of acetaminophen, an analgesic without COX-2 inhibiting properties (OR = 1.02). The inverse pattern of risk for acetaminophen, low dose aspirin, regular aspirin, ibuprofen and coxibs was significant by a linear trend test (P < 0.05) suggesting that chemopreventive effects become progressively stronger with greater selective COX-2 inhibition.
| Agent | OR (95%CI) |
| COX-2 inhibitor | 0.29 (0.14-0.59) |
| Ibuprofen | 0.37 (0.18-0.72) |
| Regular aspirin (325 mg) | 0.51 (0.27-0.98) |
| Low dose aspirin (81 mg) | 0.77 (0.41-1.41) |
| Acetaminophen | 1.02 (0.39-2.20) |
| Selective COX-2 inhibitors: Celecoxib or Rofecoxib |
During the time period 1987-2008, we conducted a series of epidemiologic studies of NSAIDs and cancers of the breast, prostate, colon and lung[57,59,61,69,79,95-99]. Five of these studies focused on cancer of the breast. In each investigation, information was obtained about the entire profile of NSAID use for each participant including both over-the-counter and prescription drugs. All studies were designed to specifically evaluate and compare the two major over-the-counter compounds, aspirin and ibuprofen. Following their FDA approval, selective COX-2 inhibiting agents were also evaluated. Effects of specific agents were quantified by estimating relative risks (or odds ratios) adjusted for cancer risk factors with standard errors and 95%CIs. In each study, estimates for specific compounds were derived by comparison with a reference group that did not report using any type of NSAID. Furthermore, acetaminophen, a commonly used analgesic with little or no activity against either COX-1 or COX-2 was always evaluated as a comparator drug. Meta-analysis as described above was applied to examine effects of individual compounds for each individual cancer and across all malignancies[100,101].
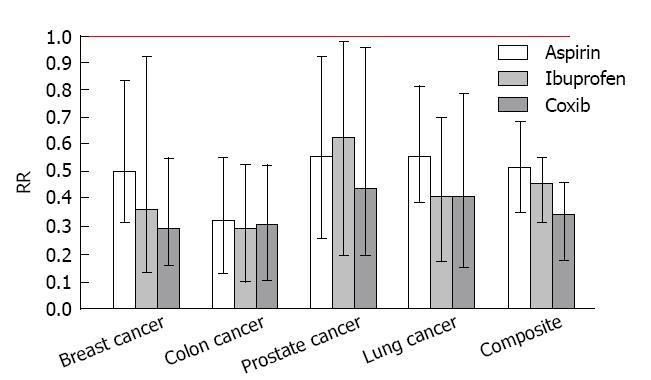
Figure 4 presents the individual and composite risk estimates for the four cancer sites with exposure to regular aspirin, ibuprofen or selective COX-2 inhibitors (celecoxib or rofecoxib). Daily intake of a selective COX-2 inhibitor (either celcoxib or rofecoxib) produced a significant reduction in the risk for each type of cancer (71% for breast cancer, 55% for prostate cancer, 70% for colon cancer, and 79% for lung cancer). The observed chemopreventive effects of coxibs were associated with recommended daily doses of celecoxib (median dose = 200 mg) or rofecoxib (median dose = 25 mg). Significant risk reductions of slightly lesser magnitude were observed for over-the-counter NSAIDs with nonselective COX-2 activity, such as regular (325 mg) aspirin and (200 mg) ibuprofen. Daily intake of baby (81 mg) aspirin produced marginally significant risk reductions for colon cancer and lung cancer, but did not significantly reduce the risk of breast cancer or prostate cancer whereas daily acetaminophen, an analgesic without COX-2 activity, did not produce a significant change in the risk of any of the cancers studied. Composite risk reductions of 64%, 53% and 46% were observed for the selective COX-2 inhibitors (either celecoxib or rofecoxib), ibuprofen and aspirin, respectively; a significant dose response pattern that is consistent with the degree of selective COX-2 blockade (celecoxib > ibuprofen > aspirin).
Notably, selective COX-2 inhibitors (celecoxib and rofecoxib) were only recently approved for use in 1999. In 2004, rofecoxib (Vioxx) was withdrawn from the marketplace due to concerns about cardiovascular risk. Nevertheless, even in the short window of exposure to these compounds, the selective COX-2 inhibitors produced significant reductions in the risk of the four major human cancers (breast, prostate, colon, and lung). It is also important to note that ibuprofen produced effects similar in magnitude to the coxibs which is consistent with its high activity against COX-2. These results tend to substantiate the important role of COX-2 in carcinogenesis, and reciprocally, the strong potential for selective COX-2 blockade in cancer chemoprevention.
Randomized clinical trials of nonselective COX-2 inhibitors such as aspirin and ibuprofen for human cancer therapy are lacking. Nevertheless, since these drugs are frequently regularly taken for pain relief in randomized clinical trials of cancer, some investigators have examined their therapeutic impact among patients.
Remarkably, the treatment-adjusted hazard ratios for NSAID users show significant reductions of recurrence risk or death in three cohorts of breast cancer patients. It is emphasized that effects of ibuprofen and aspirin estimated from these studies are adjusted for stage at cancer detection, surgical treatment, chemotherapy, radiation therapy and other prognostic indicators such as age, race and gender.
Kwan et al[102] examined the association between NSAID use and breast cancer recurrence among 2292 women diagnosed with breast cancer in the Life After Cancer Epidemiology Study. They observed that regular ibuprofen users experienced 44% less recurrence than non-users after five years of follow-up.
Blair et al[103] examined effects of NSAID intake on survival after invasive breast cancer diagnosis among 591 postmenopausal women ascertained through the Iowa Women’s Health Study. Compared to nonusers, women who regularly took an NSAID experienced a 36% reduction in breast cancer mortality and a 43% reduction in all-cause mortality after approximately 10 years of followup.
Holmes et al[104] examined effects of taking aspirin or non-aspirin NSAIDs such as ibuprofen among 4164 women presenting with invasive breast cancers in the Nurses Health Study. They found that aspirin intake after breast cancer diagnosis was associated with a decreased risk of breast cancer recurrence, death from breast cancer and death from any cause. Significant decreases in breast cancer mortality of 71% and 64% were noted for aspirin intake 2-5 times per week and 6-7 times per week, respectively. More limited results from the study suggested that daily intake of non-aspirin NSAIDs also reduced breast cancer mortality whereas acetaminophen use showed no evidence of survival benefit.
White adipocytes are intimately and inseparably connected to the parenchyma of the human female breast throughout life[105]. These cells provide the nutrients essential for the morphogenesis, maturation and function of the mammary epithelium. Homeostasis of the breast epithelium therefore depends vitally upon the integrity of the adipocyte population of the mammary gland. Far from being an inert fat storage depot and energy resource for parenchymal cells (e.g., the mammary epithelium), white adipose tissue is an active endocrine organ that secretes a variety of bioactive proteins collectively called adipokines[106].
Recent data from the World Health Organization and the International Agency for Research on Cancer reflects a 20% increase in the global incidence of breast cancer and a 14% increase in breast cancer mortality during the past five years[107]. These increases are most likely largely attributable to the global pandemic of obesity that influences breast cancer development and progression.
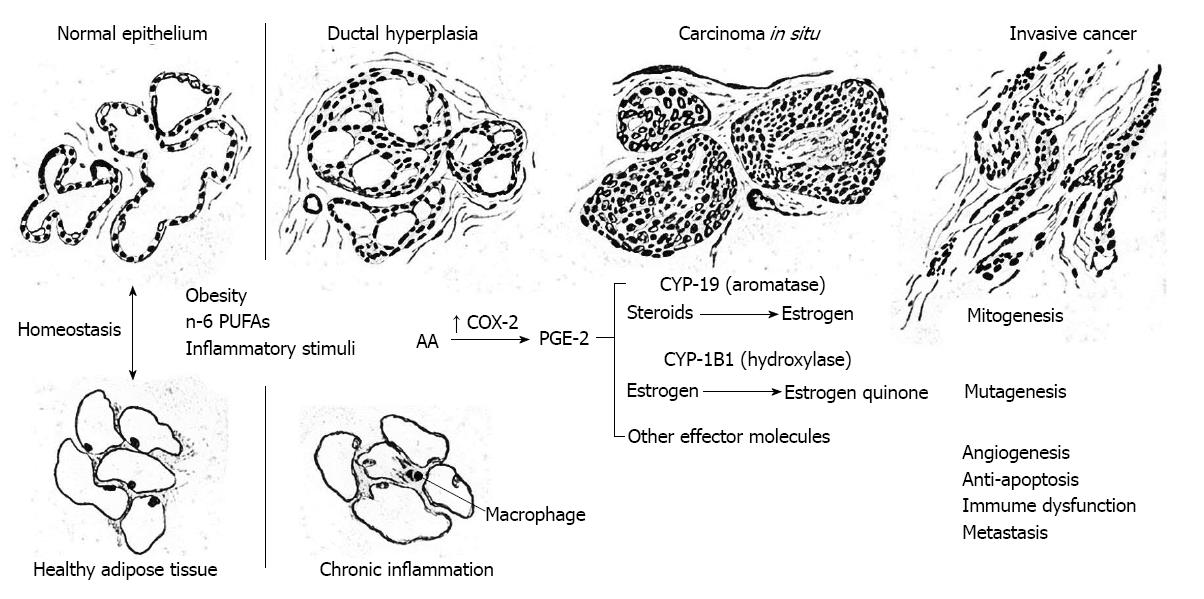
In molecular studies of tissues from humans and animals, obesity leads to inflammation and infiltration of mammary and visceral adipose tissue by macrophages with activation of NF-κB, overexpression of COX-2 and hypersecretion of PGE-2 and pro-inflammatory mediators and adipokines such as leptin, resistin, IL-6, IL-1β and tumor necrosis factors (TNF)-α[108-111]. Furthermore, COX-2 driven PGE-2 biosynthesis induces transcription of CYP-19 and aromatase-catalyzed production of estrogen in a paracrine mechanism. Local estrogen biosynthesis in the breast parenchyma has been hypothesized to be a key feature of breast cancer development, particularly in postmenopausal women[112,113].
Various molecular mechanisms may be responsible for the initiation and promotion of mammary carcinogenesis by COX-2. It is indeed remarkable that the induction of constitutive COX-2 expression and PGE2 biosynthesis are sufficient to stimulate all of the key features of mammary carcinogenesis including mutagenesis, mitogenesis, angiogenesis, metastasis, inhibition of apoptosis and immunosupression with reduced antineoplastic activity of T and B lymphocytes. These mechanisms are thoroughly reviewed and discussed elsewhere, e.g., Shiff et al[114] and Subbaramaiah et al[115].
As depicted in Figure 5, continuous over-expression of COX-2 can initiate and promote carcinogenesis by (1) increasing production of PGE-2 and other prostaglandins that strongly promote cell proliferation, e.g., correlative up-regulation of the gene for aromatase (CYP19) and estrogen biosynthesis in stromal cells, or activation of epidermal growth factor receptor (EGFR) that stimulate an intracellular cascade of mitogenic signaling (mitogenesis); (2) increasing production of estrogen quinones and other reactive oxygen species, e.g., malondialdehyde, that are carcinogenic (mutagenesis); (3) stimulation of vascular endothelial growth factor (VEGF) and platelet derived growth factor by PGE-2 resulting in de novo formation of blood vessels (angiogenesis); (4) increasing production of matrix metalloproteinases (MMP) via co-expression of COX-2 and the Her-2/Neu gene, thus enhancing invasive potential (metastasis); (5) stimulating telomerase expression, decreasing bioavailable arachidonic acid pools necessary for conversion of sphingomyelin to ceramide, and stimulation of the Bcl-2 gene and inhibition of the BAX gene thereby reducing cell differentation and apoptosis (anti-apoptosis); and (6) inhibiting proliferation of B and T lymphocytes, particularly natural killer T cells, thus limiting antineoplastic activity (immunosuppression). All of these processes are discussed in some detail below.
A key event in the carcinogenic process is induction of constitutive expression of the COX-2 gene. Molecular studies from multiple laboratories reveal that adenocarcinoma of the breast is characterized by aberrant over-expression of COX-2 by breast cancer epithelial cells[24,33-38]. As shown by Karmali et al[116] Rose et al[117], and others[33-38], arachidonic acid production, COX-2 expression, and prostaglandin biosynthesis are increased in vivo by dietary n-6 polyunsaturated fatty acids (n-6-PUFAs) such as unconjugated linoleic acid, and decreased by n-3-PUFAs such as linolenic acid. High dietary intake of n-6-PUFAs may therefore be an important factor in the induction of constitutive COX-2 expression. This mechanism is compatible with the high rates of cancers of the breast, colon and prostate (neoplasms that characteristically over-express COX-2) in populations where n-6-PUFAs, particularly unconjugated linoleic acid, are abundant in the diet. The COX-2 enzyme efficiently catalyzes the conversion of essential dietary fats (principally arachidonic acid and unconjugated linoleic acid) into prostaglandins[100,113,118].
Obesity has reached epidemic proportions in most industrialized nations in association with increased rates of a variety of chronic conditions such as type 2 diabetes, coronary heart disease and certain malignant neoplasms including breast cancer. The obese phenotype is characterized by the presence of fat-laden adipocytes that secrete pro-inflammatory adipokines (e.g., leptin and resistin) and stimulate infiltration of the mammary fat by macrophages which secrete pro-inflammatory cytokines (e.g., IL-6 and TNF-α). Since the COX-2 gene contains multiple promoter binding sites, these effector molecules may also participate in signal transduction cascades to induce constitutive overexpression of COX-2 and PGE-2 biosynthesis[45,108-111,114,115]. Furthermore, in vitro studies of breast cancer tissues suggest that mutations or methylation of CpG islands at binding sites for these transcription factors in the promoter region of the COX-2 gene regulate the induction of COX-2 transcription[119]. Thus, induction of constitutive COX-2 genetic expression may involve synergistic interactions between a number of micro-environmental epigenetic and genetic cofactors.
The COX-2 enzyme efficiently catalyzes the conversion of essential dietary fats (principally arachidonic acid and unconjugated linoleic acid) into prostaglandins. Induction of constitutive over-expression of COX-2 in a cell predominantly increases the biosynthesis of PGE-2, which is the chief prostaglandin of the inflammatory cascade. This short-lived intercellular hormone is capable of inducing the transcription of specific genes in the nucleus of nearby cells. In particular, PGE-2 has been found to stimulate the transcription of genes that have powerful mitogenic effects.
Importantly, it has recently been discovered that there is a strong link between prostaglandins and a paracrine mechanism of estrogen biosynthesis. This occurs when the chief prostaglandin, PGE-2, activates the promoter II region of the aromatase gene (CYP-19), which is responsible for local estrogen biosynthesis catalyzed by aromatase[112]. Furthermore, several molecular studies have revealed a significant correlation between up-regulation of cyclooxygenase expression and CYP-19 transcription and aromatase-catalyzed estrogen biosynthesis in breast cancer tissues[108,109,120-122]. Notably, this mechanism has been demonstrated in other malignant neoplasms including cancers of the lung, colon, and prostate and may in fact be a ubiquitous feature in cancer promotion and development[123-130]. Clearly, the established molecular link between heightened levels of n-6 polyunsaturated fatty acids, COX-2, PGE-2, aromatase and estrogens, provides a basis for mammary carcinogenesis through unbridled mitogenesis.
An additional mitogenic mechanism is PGE-2 activation of EGFR that in turn triggers cell division through the mitogen-activated protein kinase (MAPK) cascade[131-133]. Polakis et al[134] discovered that PGE-2 rapidly phosphorylates EGFR and triggers the extracellular kinase, ERK-2, thereby activating the mitogenic signaling cascade in normal gastric epithelium and colon cancer. Their studies indicate that PGE-2-induced EGFR transactivation involves signal transduction via TGF-α and activated MMP. Other investigators have confirmed that co-expression of COX-2, PGE-2, and EGFR results in mitogenic activation in precancerous and cancerous tissues of multiple anatomic sites[135-139]. In a recent molecular study of COX-2 and EGFR in human breast cancer tissues from 55 patients, COX-2 expression was detected in cancer cells of more than 95% of specimens and EGFR expression was found to be dependent on COX-2 upregulation[140].
Other COX-2 driven mechanisms may also be involved in delimiting cell proliferation of the ductal epithelium of mammary tissues. For example, PGE-2 expression is associated with disruption of contact inhibition in malignant cells from specimens of cancerous tissues from multiple anatomic sites.
Molecular examination of colon cancer specimens first revealed accumulation of the cell adhesion molecule, beta-catenin, in the nucleus of malignant cells[133-136]. Cell adhesion is under the control of the gene for adenomatous polyposis coli (APC) and involves maintenance of the integrity of a molecular cell adhesion complex comprised of beta-catenin, APC protein, T-cell factor and actin. Familial adenomatous polyposis is caused by a mutation of the APC gene that causes dissociation of these cell adhesion complexes and the migration of beta catenin to the cell nucleus where it activates one of the peroxisome proliferator activated receptors (PPAR gamma) on the nuclear membrane. Castellone et al[137] conducted a series of experiments demonstrating that inhibition of PGE-2 biosynthesis by NSAIDs effectively reduces the accumulation of beta-catenin and the progression of colon cancer. Based on their results, over-expression of COX-2 with increased PGE-2 biosynthesis and binding to its receptor in turn activates a cytoplasmic G-protein receptor that binds axin thereby reducing phosphorylation of beta-catenin. This chain of molecular events leads to dissociation of the adhesion complex, accumulation of unphosphorylated beta-catenin in the cell nucleus, activation of the nuclear receptor, PPAR-gamma, and stimulation of cell proliferation through transcription of cell cyclin genes. Recent molecular studies suggest that this mechanism is not limited to the colon; that is, induction of cyclooxygenase and increased PGE-2 can result in cellular beta-catenin accumulation, nuclear PPAR-gamma activation, and subsequent cell proliferation and carcinogenesis in a variety of tissues including the mammary epithelium[138,139].
Accumulated mutagenic damage to DNA is believed to contribute substantially to the etiology of breast cancer. Notably, there is strong experimental evidence to support the role of estrogen metabolites as carcinogenic agents. Specifically, the metabolism of estrogens by certain enzymes of the cytochrome P450 system produces catechol estrogens that can be further oxidized to form quinones that react directly with DNA or undergo redox cycling to generate reactive oxygen species that cause oxidative damage to DNA. This mechanism is of major significance in chronically inflamed breast tissue (e.g., in obese women) wherein PGE-2 activates aromatase-catalyzed estrogen biosynthesis.
The quinone metabolites of estrogen are formed by the action of the same cytochrome P450 enzymes responsible for the metabolism of polycyclic aromatic hydrocarbones (cytochrome P450 isoforms CYP-1A1, CYP-1B1, and CYP-3A). While most P450 enzymes are produced in the liver, CYP-1B1 is constitutively expressed in the mammary gland and other extrahepatic tissues. In the mammary gland, CYP-1B1 preferentially metabolizes estrogen to 4-hydroxyestrogen which is oxidized to form carcinogenic 3,4 estrogen quinone which in turn forms unstable adducts with adenine and guanine in DNA, leading to depurination and mutation in vitro and in vivo. Reduction of estrogen quinones to hydroquinones and catechols can also form reactive oxygen species by redox recycling[140-147].
Another established mechanism of mutagenesis involves constitutive COX-2 expression and intermediate compounds formed by activation of the prostaglandin cascade. It is well known that lipid peroxidation in the human system generates reactive electrophilic compounds that have mutagenic potential. COX catalyze the two-step oxidation and peroxidation of arachidonic acid to form the intermediate prostaglandin endoperoxides, PGG-2 and PGH-2[13,14,45,114,115,148]. Spontaneous breakdown of PGH-2 yields the mutagen, malondialdehyde (MDA) plus hydroxyheptadecatreionic acid, and specific enzymes of the cytochrome P450 system as well as thromboxane synthetase can also catalyze the breakdown of PGH-2 to MDA[149]. Malondialdehyde reacts with DNA under physiological conditions to form DNA adducts, predominantly pyrimidopurinone adducts of deoxyguanosine[150]. Sharma et al[151] demonstrated that induction of COX-2 in human non-malignant colon epithelial cells produced increases in PGE-2, MDA, and characteristic DNA adducts that were similar to the levels observed in malignant colon epithelial cells. These findings underscore the potential for carcinogenesis due to oxidative damage and mutagenesis attributable to constitutive over-expression of COX-2.
VEGF is a potent stimulant of de novo blood vessel formation (angiogenesis) in a variety of tissues. Once believed present only in the endothelial lining of blood vessels, VEGF has now been discovered in virtually all types of cancers[152,153]. Molecular investigations of breast cancer tissues provide strong evidence that COX-2-derived PGE-2 stimulates the synthesis and release of VEGF resulting in angiogenesis and ingrowth of new blood vessels that are immature and highly permeable thereby facilitating metastatic spread of tumor cells[7,28,154]. Tumor secretion of VEGF (and other growth factors) may further amplify COX-2 expression in a positive feedback loop to produce lymphangiogenesis[155,156]. Notably, inhibition of this vicious cycle by COX-2 inhibiting agents such as celecoxib has been found to limit angiogenesis and halt the progression and metastatic spread of tumors in animals[157].
Apoptosis or controlled cell death is an important regulatory mechanism for the maintenance of homeostasis in cell populations. Dysfunctional apoptosis results in immortalization of cells, a key feature of cancer cells. Inflammation, COX-2 over-expression, and increased PGE-2 are clearly anti-apoptotic, whereas, anti-inflammatory compounds that inhibit COX-2 are pro-apoptotic[21,22,114,115,158].
Apoptosis is regulated by an intrinsic pathway that originates inside the cell and an extrinsic pathway that originates outside the cell and in molecular studies of breast cancer tissues, both pathways are inhibited by COX-2 over-expression[159-161]. The intrinsic pathway involves mitochondrial release of cytochrome c and activation of caspase 9 and other enzymes that destroy the cell. Intrinsic apoptosis is triggered when the expression of two nuclear genes, Bcl-2 and BAX, favors BAX. Notably, COX-2 over-expression and prostaglandin biosynthesis promotes Bcl-2 and inhibits BAX, thereby blocking intrinsic apoptosis[159,160].
The extrinsic pathway involves activation of death receptors on the cell membrane by TNF-α, -β and other epigenetic factors. This results in activation of caspase 8 and other enzymes that destroy the cell. Over-expression of COX-2 attenuates activation of this mechanism thereby blocking extrinsic apoptosis[161].
Compounds that inhibit COX-2 and PGE-2 appear to enhance both intrinsic and extrinsic apoptosis and as a consequence, COX-2 inhibitors used in combination with radiation show beneficial synergism in the elimination of cancer cells in inoperable solid tumors[162,163]. Nonsteroidal anti-inflammatory drugs have also been found to increase apoptosis by other mechanisms, e.g., by increasing bioavailable arachidonic acid pools necessary for conversion of sphingomyelin to ceramide since ceramide accumulation in the cell triggers apoptosis[164]. In an interesting study of a breast cell line immortalized by introduction of the human telomerase gene, a selective COX-2 inhibitor, celecoxib, induced apoptosis and inhibited growth in association with upregulation of insulin-like growth factor[165].
The Her-2/Neu oncogene is a member of the EGFR family. It is an important mediator of cancer cell growth and metastasis. Koki et al[7] and Subbaramaiah et al[166] demonstrated that COX-2 and Her-2/Neu are co-expressed in breast cancer tissues. Co-expression of COX-2 and Her-2/Neu stimulate the MAPK/AP-1 signaling cascade. When the Her-2/Neu receptor protein is activated, multiple other factors are activated that promote tumor development and metastatic spread of cancer cells[7]. Overexpression of Her-2/Neu is now widely used by clinicians as a biomarker of poor prognosis and metastasis for patients with invasive breast cancer[167].
Molecular studies of breast cancer tissues have demonstrated that high levels of COX-2 and PGE-2 are correlated with amplified Her-2/Neu expression and increased activity of MMP[168,169]. The MMP are proteolytic enzymes that degrade basement membranes and are thus associated with tumor invasiveness, metastasis, and poor survival. Reciprocally, in animal models of breast cancer, agents that inhibit COX-2 or block membrane receptors of PGE-2 have been found to reduce Her-2/Neu and MMP levels thereby decreasing the metastatic potential of cancer cells[170,171].
Immunosuppression is a characteristic feature of cancer patients that correlates with disease promotion and progression. It is an interesting paradox that COX-2 over-expression and prostaglandin biosynthesis empowers cancer cell proliferation, immortalization, and metastasis on the one hand, while suppressing the function of important cells of the immune system on the other, thereby creating an immunosuppressed host with little ability to mount an immune defense against a developing tumor. Indeed, the induction of T cell anergy is an early event in the course of tumor progression[172].
Prostaglandins, particularly PGE-2, are important modulators of immunosuppression. Pockaj et al[173] found that increased levels of PGE-2 suppress the immunocompetence of helper T-cells and dendritic cells in newly diagnosed breast cancer patients. Specifically, elevated levels of PGE-2 were associated with reduced secretion of antitumor factors by T-cells (interferon-gamma, TNF-alpha, and interleukins IL-2 and IL-12) and loss of immunocompetence in dendritic cells (reduced secretion of stimulatory molecules, loss of antigen-sensitizing function, reduced phagocytic activity, and lack of maturation potential). Defective T-cell and dendritic cell function due to COX-2 driven PGE-2 biosynthesis is therefore an important mechanism by which tumors evade immunosurveillance.
It should be emphasized that the proposed “inflammogenesis model of breast cancer” is not mutually exclusive and may in fact be synergistic with other mechanisms of mammary carcinogenesis. For example, polycyclic aromatic hydrocarbons and other carcinogens present in tobacco smoke are mutagenic in mammary tissues[174] and acetaldehyde, the primary metabolite of alcohol metabolism has powerful mutagenic impact in all tissues studied[175]. The web of breast cancer causation may thus be particularly strong in obese women who are chronically addicted to both tobacco and alcohol and regularly consume diets with a high content of n-6 PUFA.
Cohesive scientific evidence from molecular, animal, and human investigations supports the hypothesis that induction of constitutive COX-2 over-expression and upregulation of the prostaglandin cascade play a significant role in mammary carcinogenesis, and reciprocally, blockade of the process has strong potential for breast cancer prevention and therapy. A summary of the evidence supporting the “inflammogenesis of breast cancer” is given below: (1) Epidemiologic investigations have consistently demonstrated that nonselective COX-2 inhibitors, such as aspirin and ibuprofen, used on a regular basis, significantly reduce the risk of human breast cancer; (2) Selective COX-2 inhibitors, such as celecoxib, used on a regular basis have been shown to reduce the risk of human breast cancer; (3) Follow-up studies of women with breast cancer have consistently demonstrated that nonselective COX-2 inhibitors significantly reduce recurrence risk and breast cancer mortality; (4) Molecular investigations show that COX-2 expression is a characteristic feature of premalignant mammary neoplasms and ductal carcinoma in situ; (5) Molecular investigations show that COX-2 expression is a characteristic feature of invasive breast cancer and expression tends to intensify with stage at detection and cancer progression and metastasis; (6) All essential features of carcinogenesis (mitogenesis, mutagenesis, angiogenesis, reduced apoptosis, metastasis, and immunosuppression) are linked to COX-2-driven PGE-2 biosynthesis; and (7) Most notably, upregulation of COX-2 and PGE-2 expression induces transcription of CYP-19 and aromatase-catalyzed estrogen biosynthesis by the mammary adipose tissue which stimulates unbridled mitogenesis of ductal epithelium, and the extrahepatic mammary enzyme, CYP-1B1, converts paracrine estrogen to carcinogenic quinones that have potent mutagenic impact.
This review documents compelling evidence that mammary carcinogenesis often evolves as a progressive series of highly specific cellular and molecular changes in response to induction of constitutive over-expression of COX-2 and the prostaglandin cascade in the “inflammogenesis of cancer”. Based upon results, a general model of inflammogenesis of cancer is proposed involving induction of constitutive COX-2 expression and upregulation of the prostaglandin cascade.
It is emphasized that encouraging results regarding the chemopreventive and therapeutic effects of both selective and non-selective COX-2 inhibiting agents against cancer of the breast as well as other malignant neoplasms have been tempered by concerns about cardiovascular risk associated with taking compounds that inhibit COX-2[10]. For example, data from randomized trials suggests that high dosages of some NSAIDs or combinations of such drugs may increase the risk of cardiovascular disease[176]. Before recommendations can be made, more studies are needed to determine if certain COX-2 inhibiting drugs can be taken at dosages that prevent cancer without increasing the risk of heart conditions.
P- Reviewer: Maia CJ, Shyue SK, Wieczorek E S- Editor: Wen LL L- Editor: A E- Editor: Liu SQ
| 1. | Virchow R. Reizung and Reizbarkeit. Arch Pathol Anat Klin Med. 1858;14:1-63. [Cited in This Article: ] |
| 2. | Virchow R. Aetiologie der neoplastischen Geschwulst/Pathogenie der neoplastischen Geschwulste. In: Die Krankhaften Geschwulste. 1863;57-101. [Cited in This Article: ] |
| 3. | Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539-545. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5245] [Cited by in F6Publishing: 5418] [Article Influence: 235.6] [Reference Citation Analysis (0)] |
| 4. | Schrieber H, Rowley DA. Inflammation and cancer. Inflammation: Basic Principles and Clinical Correlates, third ed. Philadelphia: Lippincott Williams & Wilkins 1999; 1117-1129. [Cited in This Article: ] |
| 5. | Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860-867. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10123] [Cited by in F6Publishing: 10557] [Article Influence: 479.9] [Reference Citation Analysis (0)] |
| 6. | Philip M, Rowley DA, Schreiber H. Inflammation as a tumor promoter in cancer induction. Semin Cancer Biol. 2004;14:433-439. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 424] [Cited by in F6Publishing: 420] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 7. | Koki AT, Leahy KM, Harmon JM, Masferrer JL. Cyclooxygenase-2 and cancer. In: RE Harris, ed. COX-2 Blockade in Cancer Prevention and Therapy. Humana Press, Totowa, NJ 2002; 185-203. [Cited in This Article: ] |
| 8. | Jang BC, Hla T. Regulation of expression and potential carcinogenic role of cylcooxygenase-2. In: RE Harris, ed. COX-2 Blockade in Cancer Prevention and Therapy. Humana Press, Totowa, NJ 2002; 171-184. [Cited in This Article: ] |
| 9. | Harris RE. Cyclooxygenase-2 blockade in cancer prevention and therapy: widening the scope of impact. In: RE Harris, ed. COX-2 Blockade in Cancer Prevention and Therapy. Humana Press, Totowa, NJ 2002; 341-365. [Cited in This Article: ] |
| 10. | Harris RE. Cyclooxygenase-2 (cox-2) and the inflammogenesis of cancer. Subcell Biochem. 2007;42:93-126. [PubMed] [Cited in This Article: ] |
| 11. | Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971;231:232-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5183] [Cited by in F6Publishing: 4888] [Article Influence: 92.2] [Reference Citation Analysis (0)] |
| 12. | Hla T, Neilson K. Human cyclooxygenase-2 cDNA. Proc Natl Acad Sci USA. 1992;89:7384-7388. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1029] [Cited by in F6Publishing: 1033] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 13. | Herschman HR. Regulation of prostaglandin synthase-1 and prostaglandin synthase-2. Cancer Metastasis Rev. 1994;13:241-256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 243] [Cited by in F6Publishing: 255] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 14. | Herschman HR. Historical Aspects of COX-2. In: RE Harris, ed. COX-2 Blockade in Cancer Prevention and Therapy. Humana Press, Totowa, NJ 2002; 13-32. [Cited in This Article: ] |
| 15. | Clària J. Cyclooxygenase-2 biology. Curr Pharm Des. 2003;9:2177-2190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 166] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 16. | Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1:a001651. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2497] [Cited by in F6Publishing: 3078] [Article Influence: 205.2] [Reference Citation Analysis (0)] |
| 17. | Gabay C. Interleukin-6 and chronic inflammation. Arthritis Res Ther. 2006;8 Suppl 2:S3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 774] [Cited by in F6Publishing: 903] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 18. | Maihöfner C, Charalambous MP, Bhambra U, Lightfoot T, Geisslinger G, Gooderham NJ. Expression of cyclooxygenase-2 parallels expression of interleukin-1beta, interleukin-6 and NF-kappaB in human colorectal cancer. Carcinogenesis. 2003;24:665-671. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 89] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Zhao Y, Usatyuk PV, Gorshkova IA, He D, Wang T, Moreno-Vinasco L, Geyh AS, Breysse PN, Samet JM, Spannhake EW. Regulation of COX-2 expression and IL-6 release by particulate matter in airway epithelial cells. Am J Respir Cell Mol Biol. 2009;40:19-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 20. | Harris RE. Cyclooxygenase-2 (cox-2) blockade in the chemoprevention of cancers of the colon, breast, prostate, and lung. Inflammopharmacology. 2009;17:55-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 234] [Cited by in F6Publishing: 228] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 21. | Wu KK. Cyclooxygenase 2 induction: molecular mechanism and pathophysiologic roles. J Lab Clin Med. 1996;128:242-245. [PubMed] [Cited in This Article: ] |
| 22. | Dubois RN, Abramson SB, Crofford L, Gupta RA, Simon LS, Van De Putte LB, Lipsky PE. Cyclooxygenase in biology and disease. FASEB J. 1998;12:1063-1073. [PubMed] [Cited in This Article: ] |
| 23. | Parrett M, Harris R, Joarder F, Ross M, Clausen K, Robertson F. Cyclooxygenase-2 gene expression in human breast cancer. Int J Oncol. 1997;10:503-507. [PubMed] [Cited in This Article: ] |
| 24. | Hwang D, Scollard D, Byrne J, Levine E. Expression of cyclooxygenase-1 and cyclooxygenase-2 in human breast cancer. J Natl Cancer Inst. 1998;90:455-460. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 439] [Cited by in F6Publishing: 462] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 25. | Soslow RA, Dannenberg AJ, Rush D, Woerner BM, Khan KN, Masferrer J, Koki AT. COX-2 is expressed in human pulmonary, colonic, and mammary tumors. Cancer. 2000;89:2637-2645. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 26. | Ristimäki A, Sivula A, Lundin J, Lundin M, Salminen T, Haglund C, Joensuu H, Isola J. Prognostic significance of elevated cyclooxygenase-2 expression in breast cancer. Cancer Res. 2002;62:632-635. [PubMed] [Cited in This Article: ] |
| 27. | Costa C, Soares R, Reis-Filho JS, Leitão D, Amendoeira I, Schmitt FC. Cyclo-oxygenase 2 expression is associated with angiogenesis and lymph node metastasis in human breast cancer. J Clin Pathol. 2002;55:429-434. [PubMed] [Cited in This Article: ] |
| 28. | Kirkpatrick K, Ogunkolade W, Elkak A, Bustin S, Jenkins P, Ghilchik M, Mokbel K. The mRNA expression of cyclo-oxygenase-2 (COX-2) and vascular endothelial growth factor (VEGF) in human breast cancer. Curr Med Res Opin. 2002;18:237-241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 66] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Davies G, Salter J, Hills M, Martin LA, Sacks N, Dowsett M. Correlation between cyclooxygenase-2 expression and angiogenesis in human breast cancer. Clin Cancer Res. 2003;9:2651-2656. [PubMed] [Cited in This Article: ] |
| 30. | Denkert C, Winzer KJ, Müller BM, Weichert W, Pest S, Köbel M, Kristiansen G, Reles A, Siegert A, Guski H. Elevated expression of cyclooxygenase-2 is a negative prognostic factor for disease free survival and overall survival in patients with breast carcinoma. Cancer. 2003;97:2978-2987. [PubMed] [Cited in This Article: ] |
| 31. | Shim JY, An HJ, Lee YH, Kim SK, Lee KP, Lee KS. Overexpression of cyclooxygenase-2 is associated with breast carcinoma and its poor prognostic factors. Mod Pathol. 2003;16:1199-1204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Shim V, Gauthier ML, Sudilovsky D, Mantei K, Chew KL, Moore DH, Cha I, Tlsty TD, Esserman LJ. Cyclooxygenase-2 expression is related to nuclear grade in ductal carcinoma in situ and is increased in its normal adjacent epithelium. Cancer Res. 2003;63:2347-2350. [PubMed] [Cited in This Article: ] |
| 33. | Singh-Ranger G, Kirkpatrick KL, Clark GM, Mokbel K. Cyclo-oxygenase-2 (COX-2) mRNA expression correlates with progesterone receptor positivity in human breast cancer. Curr Med Res Opin. 2003;19:131-134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 34. | Boland GP, Butt IS, Prasad R, Knox WF, Bundred NJ. COX-2 expression is associated with an aggressive phenotype in ductal carcinoma in situ. Br J Cancer. 2004;90:423-429. [PubMed] [Cited in This Article: ] |
| 35. | Tan KB, Yong WP, Putti TC. Cyclooxygenase-2 expression: a potential prognostic and predictive marker for high-grade ductal carcinoma in situ of the breast. Histopathology. 2004;44:24-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 36. | Nakopoulou L, Mylona E, Papadaki I, Kapranou A, Giannopoulou I, Markaki S, Keramopoulos A. Overexpression of cyclooxygenase-2 is associated with a favorable prognostic phenotype in breast carcinoma. Pathobiology. 2005;72:241-249. [PubMed] [Cited in This Article: ] |
| 37. | Perrone G, Santini D, Vincenzi B, Zagami M, La Cesa A, Bianchi A, Altomare V, Primavera A, Battista C, Vetrani A. COX-2 expression in DCIS: correlation with VEGF, HER-2/neu, prognostic molecular markers and clinicopathological features. Histopathology. 2005;46:561-568. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 38. | Takeshita E, Osanai T, Higuchi T, Soumaoro LT, Sugihara K. Elevated cyclooxygenase-2 expression is associated with histological grade in invasive ductal breast carcinoma. J Med Dent Sci. 2005;52:189-193. [PubMed] [Cited in This Article: ] |
| 39. | Barnes N, Haywood P, Flint P, Knox WF, Bundred NJ. Survivin expression in in situ and invasive breast cancer relates to COX-2 expression and DCIS recurrence. Br J Cancer. 2006;94:253-258. [PubMed] [Cited in This Article: ] |
| 40. | Gunnarsson C, Jansson A, Holmlund B, Ferraud L, Nordenskjöld B, Rutqvist LE, Skoog L, Stål O. Expression of COX-2 and steroid converting enzymes in breast cancer. Oncol Rep. 2006;16:219-224. [PubMed] [Cited in This Article: ] |
| 41. | Mehrotra S, Morimiya A, Agarwal B, Konger R, Badve S. Microsomal prostaglandin E2 synthase-1 in breast cancer: a potential target for therapy. J Pathol. 2006;208:356-363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 42. | Chuah BY, Putti T, Salto-Tellez M, Charlton A, Iau P, Buhari SA, Wong CI, Tan SH, Wong AL, Chan CW. Serial changes in the expression of breast cancer-related proteins in response to neoadjuvant chemotherapy. Ann Oncol. 2011;22:1748-1754. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 43. | Hartmann LC, Lingle W, Frost NH, Shaun D, Maloney RA, Vierkant V, Pankratz S, Tisty T, Degnim AC, Visscher DW. COX-2 expression in atypia: correlation with breast cancer risk. : Proc Amer Assoc Cancer Res 97th Annual Meeting 2006; Abstract No. 2353. [Cited in This Article: ] |
| 44. | Visscher DW, Pankratz VS, Santisteban M, Reynolds C, Ristimäki A, Vierkant RA, Lingle WL, Frost MH, Hartmann LC. Association between cyclooxygenase-2 expression in atypical hyperplasia and risk of breast cancer. J Natl Cancer Inst. 2008;100:421-427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 45. | Howe LR, Subbaramaiah K, Brown AM, Dannenberg AJ. Cyclooxygenase-2: a target for the prevention and treatment of breast cancer. Endocr Relat Cancer. 2001;8:97-114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 227] [Cited by in F6Publishing: 240] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 46. | Karmali RA, Marsh J, Fuchs C. Effect of omega-3 fatty acids on growth of a rat mammary tumor. J Natl Cancer Inst. 1984;73:457-461. [PubMed] [Cited in This Article: ] |
| 47. | Karmali RA. Dietary fatty acids, COX-2 blockade, and carcinogenesis. In: RE Harris, ed. COX-2 Blockade in Cancer Prevention and Therapy. Humana Press, Totowa, NJ 2002; 3-12. [Cited in This Article: ] |
| 48. | Joarder F, Abouissa H, Robertson F, Parrett M, Alshafie G, Harris R. Growth arrest of DMBA-induced mammary carcinogenesis with ibuprofen treatment in female Sprague-Dawley rats. Oncol Rep. 1997;4:1271-1273. [PubMed] [Cited in This Article: ] |
| 49. | Robertson FM, Parrett ML, Joarder FS, Ross M, Abou-Issa HM, Alshafie G, Harris RE. Ibuprofen-induced inhibition of cyclooxygenase isoform gene expression and regression of rat mammary carcinomas. Cancer Lett. 1998;122:165-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 54] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 50. | Abou-Issa HM, Alshafie GA, Harris RE. Chemoprevention of breast cancer by nonsteroidal anti-inflammatory drugs and selective COX-2 blockade in animals. In: RE Harris, ed. COX-2 Blockade in Cancer Prevention and Therapy. Humana Press, Totowa, NJ 2002; 85-98. [Cited in This Article: ] |
| 51. | Harris RE, Alshafie GA, Abou-Issa H, Seibert K. Chemoprevention of breast cancer in rats by celecoxib, a cyclooxygenase 2 inhibitor. Cancer Res. 2000;60:2101-2103. [PubMed] [Cited in This Article: ] |
| 52. | Liu CH, Chang SH, Narko K, Trifan OC, Wu MT, Smith E, Haudenschild C, Lane TF, Hla T. Overexpression of cyclooxygenase-2 is sufficient to induce tumorigenesis in transgenic mice. J Biol Chem. 2001;276:18563-18569. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 579] [Cited by in F6Publishing: 558] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 53. | Paganini-Hill A, Chao A, Ross RK, Henderson BE. Aspirin use and chronic diseases: a cohort study of the elderly. BMJ. 1989;299:1247-1250. [PubMed] [Cited in This Article: ] |
| 54. | Thun MJ, Namboodiri MM, Calle EE, Flanders WD, Heath CW. Aspirin use and risk of fatal cancer. Cancer Res. 1993;53:1322-1327. [PubMed] [Cited in This Article: ] |
| 55. | Schreinemachers DM, Everson RB. Aspirin use and lung, colon, and breast cancer incidence in a prospective study. Epidemiology. 1994;5:138-146. [PubMed] [Cited in This Article: ] |
| 56. | Harris RE, Namboodiri K, Stellman SD, Wynder EL. Breast cancer and NSAID use: heterogeneity of effect in a case-control study. Prev Med. 1995;24:119-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 48] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 57. | Harris R, Namboodiri K, Farrar W. Epidemiologic-study of nonsteroidal antiinflammatory drugs and breast-cancer. Oncol Rep. 1995;2:591-592. [PubMed] [Cited in This Article: ] |
| 58. | Rosenberg L. Nonsteroidal anti-inflammatory drugs and cancer. Prev Med. 1995;24:107-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 53] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 59. | Harris RE, Namboodiri KK, Farrar WB. Nonsteroidal antiinflammatory drugs and breast cancer. Epidemiology. 1996;7:203-205. [PubMed] [Cited in This Article: ] |
| 60. | Egan KM, Stampfer MJ, Giovannucci E, Rosner BA, Colditz GA. Prospective study of regular aspirin use and the risk of breast cancer. J Natl Cancer Inst. 1996;88:988-993. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 128] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 61. | Harris RE, Kasbari S, Farrar WB. Prospective study of nonsteroidal anti-inflammatory drugs and breast cancer. Oncol Rep. 1999;6:71-73. [PubMed] [Cited in This Article: ] |
| 62. | Johnson TJ, Anderson KI, Lazovich D, Folsom AR. Association of aspirin and other nonsteroidal anti-inflammatory drug use with incidence of postmenopausal breast cancer. Proc Amer Assoc Cancer Res. 2001;42:Abstract 4098: 763. [Cited in This Article: ] |
| 63. | Neugut AI, Rosenberg DJ, Ahsan H, Jacobson JS, Wahid N, Hagan M, Rahman MI, Khan ZR, Chen L, Pablos-Mendez A. Association between coronary heart disease and cancers of the breast, prostate, and colon. Cancer Epidemiol Biomarkers Prev. 1998;7:869-873. [PubMed] [Cited in This Article: ] |
| 64. | Coogan PF, Rao SR, Rosenberg L, Palmer JR, Strom BL, Zauber AG, Stolley PD, Shapiro S. The relationship of nonsteroidal anti-inflammatory drug use to the risk of breast cancer. Prev Med. 1999;29:72-76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 70] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 65. | Sharpe CR, Collet JP, McNutt M, Belzile E, Boivin JF, Hanley JA. Nested case-control study of the effects of non-steroidal anti-inflammatory drugs on breast cancer risk and stage. Br J Cancer. 2000;83:112-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 131] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 66. | Cotterchio M, Kreiger N, Sloan M, Steingart A. Nonsteroidal anti-inflammatory drug use and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2001;10:1213-1217. [PubMed] [Cited in This Article: ] |
| 67. | Langman MJ, Cheng KK, Gilman EA, Lancashire RJ. Effect of anti-inflammatory drugs on overall risk of common cancer: case-control study in general practice research database. BMJ. 2000;320:1642-1646. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 303] [Cited by in F6Publishing: 328] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 68. | Meier CR, Schmitz S, Jick H. Association between acetaminophen or nonsteroidal antiinflammatory drugs and risk of developing ovarian, breast, or colon cancer. Pharmacotherapy. 2002;22:303-309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 65] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 69. | Harris RE, Chlebowski RT, Jackson RD, Frid DJ, Ascenseo JL, Anderson G, Loar A, Rodabough RJ, White E, McTiernan A. Breast cancer and nonsteroidal anti-inflammatory drugs: prospective results from the Women’s Health Initiative. Cancer Res. 2003;63:6096-6101. [PubMed] [Cited in This Article: ] |
| 70. | Moorman PG, Grubber JM, Millikan RC, Newman B. Association between non-steroidal anti-inflammatory drugs (NSAIDs) and invasive breast cancer and carcinoma in situ of the breast. Cancer Causes Control. 2003;14:915-922. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 71. | Garcia Rodriguez LA, Gonzalez-Perez AG. Risk of breast cancer among users of aspirin and other anti-inflammatory drugs. Brit J Cancer. 2004;91:535-539. [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 72] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 72. | Terry MB, Gammon MD, Zhang FF, Tawfik H, Teitelbaum SL, Britton JA, Subbaramaiah K, Dannenberg AJ, Neugut AI. Association of frequency and duration of aspirin use and hormone receptor status with breast cancer risk. JAMA. 2004;291:2433-2440. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 211] [Cited by in F6Publishing: 221] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 73. | Swede H, Mirand AL, Menezes RJ, Moysich KB. Association of regular aspirin use and breast cancer risk. Oncology. 2005;68:40-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 74. | Cook NR, Lee IM, Gaziano JM, Gordon D, Ridker PM, Manson JE, Hennekens CH, Buring JE. Low-dose aspirin in the primary prevention of cancer: the Women’s Health Study: a randomized controlled trial. JAMA. 2005;294:47-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 576] [Cited by in F6Publishing: 565] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 75. | Rahme E, Ghosn J, Dasgupta K, Rajan R, Hudson M. Association between frequent use of nonsteroidal anti-inflammatory drugs and breast cancer. BMC Cancer. 2005;5:159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 76. | Jacobs EJ, Thun MJ, Connell CJ, Rodriguez C, Henley SJ, Feigelson HS, Patel AV, Flanders WD, Calle EE. Aspirin and other nonsteroidal anti-inflammatory drugs and breast cancer incidence in a large U.S. cohort. Cancer Epidemiol Biomarkers Prev. 2005;14:261-264. [PubMed] [Cited in This Article: ] |
| 77. | Marshall SF, Bernstein L, Anton-Culver H, Deapen D, Horn-Ross PL, Mohrenweiser H, Peel D, Pinder R, Purdie DM, Reynolds P. Nonsteroidal anti-inflammatory drug use and breast cancer risk by stage and hormone receptor status. J Natl Cancer Inst. 2005;97:805-812. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 104] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 78. | Zhang Y, Coogan PF, Palmer JR, Strom BL, Rosenberg L. Use of nonsteroidal antiinflammatory drugs and risk of breast cancer: the Case-Control Surveillance Study revisited. Am J Epidemiol. 2005;162:165-170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 79. | Harris RE, Beebe-Donk J, Alshafie GA. Reduction in the risk of human breast cancer by selective cyclooxygenase-2 (COX-2) inhibitors. BMC Cancer. 2006;6:27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 160] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 80. | Shen J, Gammon MD, Terry MB, Teitelbaum SL, Neugut AI, Santella RM. Genetic polymorphisms in the cyclooxygenase-2 gene, use of nonsteroidal anti-inflammatory drugs, and breast cancer risk. Breast Cancer Res. 2006;8:R71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 81. | Slattery ML, Curtin K, Baumgartner R, Sweeney C, Byers T, Giuliano AR, Baumgartner KB, Wolff RR. IL6, aspirin, nonsteroidal anti-inflammatory drugs, and breast cancer risk in women living in the southwestern United States. Cancer Epidemiol Biomarkers Prev. 2007;16:747-755. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 82. | Kirsh VA, Kreiger N, Cotterchio M, Sloan M, Theis B. Nonsteroidal antiinflammatory drug use and breast cancer risk: subgroup findings. Am J Epidemiol. 2007;166:709-716. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 83. | Gallicchio L, McSorley MA, Newschaffer CJ, Thuita LW, Huang HY, Hoffman SC, Helzlsouer KJ. Nonsteroidal antiinflammatory drugs, cyclooxygenase polymorphisms, and the risk of developing breast carcinoma among women with benign breast disease. Cancer. 2006;106:1443-1452. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 84. | Gill JK, Maskarinec G, Wilkens LR, Pike MC, Henderson BE, Kolonel LN. Nonsteroidal antiinflammatory drugs and breast cancer risk: the multiethnic cohort. Am J Epidemiol. 2007;166:1150-1158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 85. | Ready A, Velicer CM, McTiernan A, White E. NSAID use and breast cancer risk in the VITAL cohort. Breast Cancer Res Treat. 2008;109:533-543. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 86. | Gierach GL, Lacey JV, Schatzkin A, Leitzmann MF, Richesson D, Hollenbeck AR, Brinton LA. Nonsteroidal anti-inflammatory drugs and breast cancer risk in the National Institutes of Health-AARP Diet and Health Study. Breast Cancer Res. 2008;10:R38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 87. | Friis S, Thomassen L, Sørensen HT, Tjønneland A, Overvad K, Cronin-Fenton DP, Vogel U, McLaughlin JK, Blot WJ, Olsen JH. Nonsteroidal anti-inflammatory drug use and breast cancer risk: a Danish cohort study. Eur J Cancer Prev. 2008;17:88-96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 88. | Brasky TM, Bonner MR, Moysich KB, Ambrosone CB, Nie J, Tao MH, Edge SB, Kallakury BV, Marian C, Goerlitz DS. Non-steroidal anti-inflammatory drugs (NSAIDs) and breast cancer risk: differences by molecular subtype. Cancer Causes Control. 2011;22:965-975. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 89. | Bosco JL, Palmer JR, Boggs DA, Hatch EE, Rosenberg L. Regular aspirin use and breast cancer risk in US Black women. Cancer Causes Control. 2011;22:1553-1561. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 90. | Bardia A, Olson JE, Vachon CM, Lazovich D, Vierkant RA, Wang AH, Limburg PJ, Anderson KE, Cerhan JR. Effect of aspirin and other NSAIDs on postmenopausal breast cancer incidence by hormone receptor status: results from a prospective cohort study. Breast Cancer Res Treat. 2011;126:149-155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 91. | Zhang X, Smith-Warner SA, Collins LC, Rosner B, Willett WC, Hankinson SE. Use of aspirin, other nonsteroidal anti-inflammatory drugs, and acetaminophen and postmenopausal breast cancer incidence. J Clin Oncol. 2012;30:3468-3477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 92. | Schlesselman JJ. Case Control Studies. New York: Oxford University Press 1982; . [Cited in This Article: ] |
| 93. | Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987;9:1-30. [PubMed] [Cited in This Article: ] |
| 94. | González-Pérez A, García Rodríguez LA, López-Ridaura R. Effects of non-steroidal anti-inflammatory drugs on cancer sites other than the colon and rectum: a meta-analysis. BMC Cancer. 2003;3:28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 135] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 95. | Harris RE, Beebe-Donk J, Doss H, Burr Doss D. Aspirin, ibuprofen, and other non-steroidal anti-inflammatory drugs in cancer prevention: a critical review of non-selective COX-2 blockade (review). Oncol Rep. 2005;13:559-583. [PubMed] [Cited in This Article: ] |
| 96. | Nelson JE, Harris RE. Inverse association of prostate cancer and non-steroidal anti-inflammatory drugs (NSAIDs): results of a case-control study. Oncol Rep. 2000;7:169-170. [PubMed] [Cited in This Article: ] |
| 97. | Harris RE, Beebe-Donk J, Schuller HM. Chemoprevention of lung cancer by non-steroidal anti-inflammatory drugs among cigarette smokers. Oncol Rep. 2002;9:693-695. [PubMed] [Cited in This Article: ] |
| 98. | Harris RE, Beebe-Donk J, Alshafie GA. Reduced risk of human lung cancer by selective cyclooxygenase 2 (COX-2) blockade: results of a case control study. Int J Biol Sci. 2007;3:328-334. [PubMed] [Cited in This Article: ] |
| 99. | Harris RE, Beebe-Donk J, Alshafie GA. Similar reductions in the risk of human colon cancer by selective and nonselective cyclooxygenase-2 (COX-2) inhibitors. BMC Cancer. 2008;8:237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 100. | Harris RE, Beebe-Donk J, Alshafie GA. Cancer chemoprevention by cyclooxygenase 2 (COX-2) blockade: results of case control studies. Subcell Biochem. 2007;42:193-212. [PubMed] [Cited in This Article: ] |
| 101. | Harris RE, Beebe J, Alshafie GA. Reduction in cancer risk by selective and non-selective cyclooxygenase 2 (COX-2) inhibitors. J Exp Pharm. 2012;4:91-96. [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 102. | Kwan ML, Habel LA, Slattery ML, Caan B. NSAIDs and breast cancer recurrence in a prospective cohort study. Cancer Causes Control. 2007;18:613-620. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 103. | Blair CK, Sweeney C, Anderson KE, Folsom AR. NSAID use and survival after breast cancer diagnosis in post-menopausal women. Breast Cancer Res Treat. 2007;101:191-197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 104. | Holmes MD, Chen WY, Li L, Hertzmark E, Spiegelman D, Hankinson SE. Aspirin intake and survival after breast cancer. J Clin Oncol. 2010;28:1467-1472. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 271] [Cited by in F6Publishing: 287] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 105. | Lyon CJ, Law RE, Hsueh WA. Minireview: adiposity, inflammation, and atherogenesis. Endocrinology. 2003;144:2195-2200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 528] [Cited by in F6Publishing: 503] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 106. | Nickell WB, Skelton J. Breast fat and fallacies: more than 100 years of anatomical fantasy. J Hum Lact. 2005;21:126-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 107. | Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer 2013; . [Cited in This Article: ] |
| 108. | Subbaramaiah K, Morris PG, Zhou XK, Morrow M, Du B, Giri D, Kopelovich L, Hudis CA, Dannenberg AJ. Increased levels of COX-2 and prostaglandin E2 contribute to elevated aromatase expression in inflamed breast tissue of obese women. Cancer Discov. 2012;2:356-365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 201] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 109. | Subbaramaiah K, Howe LR, Bhardwaj P, Du B, Gravaghi C, Yantiss RK, Zhou XK, Blaho VA, Hla T, Yang P. Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland. Cancer Prev Res (Phila). 2011;4:329-346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 306] [Cited by in F6Publishing: 299] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 110. | Simpson ER, Brown KA. Minireview: Obesity and breast cancer: a tale of inflammation and dysregulated metabolism. Mol Endocrinol. 2013;27:715-725. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 111. | Rose DP, Vona-Davis L. Biochemical and molecular mechanisms for the association between obesity, chronic inflammation, and breast cancer. Biofactors. 2014;40:1-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 112. | Zhao Y, Agarwal VR, Mendelson CR, Simpson ER. Estrogen biosynthesis proximal to a breast tumor is stimulated by PGE2 via cyclic AMP, leading to activation of promoter II of the CYP19 (aromatase) gene. Endocrinology. 1996;137:5739-5742. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 211] [Cited by in F6Publishing: 248] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 113. | Harris RE, Robertson FM, Abou-Issa HM, Farrar WB, Brueggemeier R. Genetic induction and upregulation of cyclooxygenase (COX) and aromatase (CYP19): an extension of the dietary fat hypothesis of breast cancer. Med Hypotheses. 1999;52:291-292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 45] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 114. | Shiff SJ, Rigas B. The role of cyclooxygenase inhibition in the antineoplastic effects of nonsteroidal antiinflammatory drugs (NSAIDs). J Exp Med. 1999;190:445-450. [PubMed] [Cited in This Article: ] |
| 115. | Subbaramaiah K, Dannenberg AJ. Cyclooxygenase 2: a molecular target for cancer prevention and treatment. Trends Pharmacol Sci. 2003;24:96-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 510] [Cited by in F6Publishing: 490] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 116. | Karmali RA, Marsh J. Antitumor activity in a rat mammary adenocarcinoma: the effect of cyclooxygenase inhibitors and immunization against prostaglandin E2. Prostaglandins Leukot Med. 1985;20:283-286. [PubMed] [Cited in This Article: ] |
| 117. | Rose DP, Connolly JM. Omega-3 fatty acids as cancer chemopreventive agents. Pharmacol Ther. 1999;83:217-244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 437] [Cited by in F6Publishing: 386] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 118. | Cakir Y, Plummer HK, Tithof PK, Schuller HM. Beta-adrenergic and arachidonic acid-mediated growth regulation of human breast cancer cell lines. Int J Oncol. 2002;21:153-157. [PubMed] [Cited in This Article: ] |
| 119. | Chow LW, Zhu L, Loo WT, Lui EL. Aberrant methylation of cyclooxygenase-2 in breast cancer patients. Biomed Pharmacother. 2005;59 Suppl 2:S264-S267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 120. | Brueggemeier RW, Quinn AL, Parrett ML, Joarder FS, Harris RE, Robertson FM. Correlation of aromatase and cyclooxygenase gene expression in human breast cancer specimens. Cancer Lett. 1999;140:27-35. [PubMed] [Cited in This Article: ] |
| 121. | Richards JA, Brueggemeier RW. Interactions of cyclooxygenase and aromatase pathways in normal and malignant breast cells. In: RE Harris, ed. COX-2 Blockade in Cancer Prevention and Therapy. Humana Press, Totowa, NJ 2002; 205-224. [Cited in This Article: ] |
| 122. | Díaz-Cruz ES, Brueggemeier RW. Interrelationships between cyclooxygenases and aromatase: unraveling the relevance of cyclooxygenase inhibitors in breast cancer. Anticancer Agents Med Chem. 2006;6:221-232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 123. | Weinberg OK, Marquez-Garban DC, Fishbein MC, Goodglick L, Garban HJ, Dubinett SM, Pietras RJ. Aromatase inhibitors in human lung cancer therapy. Cancer Res. 2005;65:11287-11291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 134] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 124. | Fiorelli G, Picariello L, Martineti V, Tonelli F, Brandi ML. Estrogen synthesis in human colon cancer epithelial cells. J Steroid Biochem Mol Biol. 1999;71:223-230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 125. | Ellem SJ, Risbridger GP. Aromatase and prostate cancer. Minerva Endocrinol. 2006;31:1-12. [PubMed] [Cited in This Article: ] |
| 126. | Pai R, Soreghan B, Szabo IL, Pavelka M, Baatar D, Tarnawski AS. Prostaglandin E2 transactivates EGF receptor: a novel mechanism for promoting colon cancer growth and gastrointestinal hypertrophy. Nat Med. 2002;8:289-293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 654] [Cited by in F6Publishing: 638] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 127. | Coffey RJ, Hawkey CJ, Damstrup L, Graves-Deal R, Daniel VC, Dempsey PJ, Chinery R, Kirkland SC, DuBois RN, Jetton TL. Epidermal growth factor receptor activation induces nuclear targeting of cyclooxygenase-2, basolateral release of prostaglandins, and mitogenesis in polarizing colon cancer cells. Proc Natl Acad Sci USA. 1997;94:657-662. [PubMed] [Cited in This Article: ] |
| 128. | Mestre JR, Subbaramaiah K, Sacks PG, Schantz SP, Tanabe T, Inoue H, Dannenberg AJ. Retinoids suppress epidermal growth factor-induced transcription of cyclooxygenase-2 in human oral squamous carcinoma cells. Cancer Res. 1997;57:2890-2895. [PubMed] [Cited in This Article: ] |
| 129. | Kinoshita T, Takahashi Y, Sakashita T, Inoue H, Tanabe T, Yoshimoto T. Growth stimulation and induction of epidermal growth factor receptor by overexpression of cyclooxygenases 1 and 2 in human colon carcinoma cells. Biochim Biophys Acta. 1999;1438:120-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 79] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 130. | Moraitis D, Du B, De Lorenzo MS, Boyle JO, Weksler BB, Cohen EG, Carew JF, Altorki NK, Kopelovich L, Subbaramaiah K. Levels of cyclooxygenase-2 are increased in the oral mucosa of smokers: evidence for the role of epidermal growth factor receptor and its ligands. Cancer Res. 2005;65:664-670. [PubMed] [Cited in This Article: ] |
| 131. | Dannenberg AJ, Lippman SM, Mann JR, Subbaramaiah K, DuBois RN. Cyclooxygenase-2 and epidermal growth factor receptor: pharmacologic targets for chemoprevention. J Clin Oncol. 2005;23:254-266. [PubMed] [Cited in This Article: ] |
| 132. | Bhattacharjee RN, Timoshenko AV, Cai J, Lala PK. Relationship between cyclooxygenase-2 and human epidermal growth factor receptor 2 in vascular endothelial growth factor C up-regulation and lymphangiogenesis in human breast cancer. Cancer Sci. 2010;101:2026-2032. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 133. | Whelan J, McEntee MF. Nonsteroidal anti-inflammatory drugs, prostaglandins, and APC-driven intestinal tumorigenesis. In: RE Harris, ed. COX-2 Blockade in Cancer Prevention and Therapy. Humana Press, Totowa, NJ 2002; 117-145. [Cited in This Article: ] |
| 134. | Polakis P. The oncogenic activation of beta-catenin. Curr Opin Genet Dev. 1999;9:15-21. [PubMed] [Cited in This Article: ] |
| 135. | Henderson BR. Nuclear-cytoplasmic shuttling of APC regulates beta-catenin subcellular localization and turnover. Nat Cell Biol. 2000;2:653-660. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 381] [Cited by in F6Publishing: 376] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 136. | Clevers H. Colon cancer--understanding how NSAIDs work. N Engl J Med. 2006;354:761-763. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 137. | Castellone MD, Teramoto H, Williams BO, Druey KM, Gutkind JS. Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-beta-catenin signaling axis. Science. 2005;310:1504-1510. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 695] [Cited by in F6Publishing: 693] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 138. | He TC. Association of COX-2 and PPARs in carcinogenesis and chemoprevention. In: RE Harris, ed. COX-2 Blockade in Cancer Prevention and Therapy. Humana Press, Totowa, NJ 2002; 225-244. [Cited in This Article: ] |
| 139. | Bocca C, Ievolella M, Autelli R, Motta M, Mosso L, Torchio B, Bozzo F, Cannito S, Paternostro C, Colombatto S. Expression of Cox-2 in human breast cancer cells as a critical determinant of epithelial-to-mesenchymal transition and invasiveness. Expert Opin Ther Targets. 2014;18:121-135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 140. | Liehr JG. Hormone-associated cancer: mechanistic similarities between human breast cancer and estrogen-induced kidney carcinogenesis in hamsters. Environ Health Perspect. 1997;105 Suppl 3:565-569. [PubMed] [Cited in This Article: ] |
| 141. | Yager JD. Endogenous estrogens as carcinogens through metabolic activation. J Natl Cancer Inst Monogr. 2000;67-73. [PubMed] [Cited in This Article: ] |
| 142. | Cavalieri EL, Rogan EG, Chakravarti D. Initiation of cancer and other diseases by catechol ortho-quinones: a unifying mechanism. Cell Mol Life Sci. 2002;59:665-681. [PubMed] [Cited in This Article: ] |
| 143. | Yue W, Santen RJ, Wang JP, Li Y, Verderame MF, Bocchinfuso WP, Korach KS, Devanesan P, Todorovic R, Rogan EG. Genotoxic metabolites of estradiol in breast: potential mechanism of estradiol induced carcinogenesis. J Steroid Biochem Mol Biol. 2003;86:477-486. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 137] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 144. | Rogan EG, Badawi AF, Devanesan PD, Meza JL, Edney JA, West WW, Higginbotham SM, Cavalieri EL. Relative imbalances in estrogen metabolism and conjugation in breast tissue of women with carcinoma: potential biomarkers of susceptibility to cancer. Carcinogenesis. 2003;24:697-702. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 143] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 145. | Hurh YJ, Chen ZH, Na HK, Han SY, Surh YJ. 2-Hydroxyestradiol induces oxidative DNA damage and apoptosis in human mammary epithelial cells. J Toxicol Environ Health A. 2004;67:1939-1953. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 146. | Xue W, Warshawsky D. Metabolic activation of polycyclic and heterocyclic aromatic hydrocarbons and DNA damage: a review. Toxicol Appl Pharmacol. 2005;206:73-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 629] [Cited by in F6Publishing: 579] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 147. | Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354:270-282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1250] [Cited by in F6Publishing: 1227] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 148. | Marnett LJ. Lipid peroxidation-DNA damage by malondialdehyde. Mutat Res. 1999;424:83-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 830] [Cited by in F6Publishing: 934] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 149. | Plastaras JP, Guengerich FP, Nebert DW, Marnett LJ. Xenobiotic-metabolizing cytochromes P450 convert prostaglandin endoperoxide to hydroxyheptadecatrienoic acid and the mutagen, malondialdehyde. J Biol Chem. 2000;275:11784-11790. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 61] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 150. | Marnett LJ, Basu AK, O’Hara SM, Weller PE, Rahman AFMM, Oliver JP. Reaction of malondialdehyde with guanine nucleosides: formation of adducts containing oxadraza-bicyclononene residues in the base-pairing region. J Am Chem Soc. 1986;108:1348-1350. [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 60] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 151. | Sharma RA, Gescher A, Plastaras JP, Leuratti C, Singh R, Gallacher-Horley B, Offord E, Marnett LJ, Steward WP, Plummer SM. Cyclooxygenase-2, malondialdehyde and pyrimidopurinone adducts of deoxyguanosine in human colon cells. Carcinogenesis. 2001;22:1557-1560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 242] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 152. | Folkman J. Angiogenesis. Annu Rev Med. 2006;57:1-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 980] [Cited by in F6Publishing: 962] [Article Influence: 53.4] [Reference Citation Analysis (0)] |
| 153. | Masferrer JL, Leahy KM, Koki AT, Zweifel BS, Settle SL, Woerner BM, Edwards DA, Flickinger AG, Moore RJ, Seibert K. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res. 2000;60:1306-1311. [PubMed] [Cited in This Article: ] |
| 154. | Wang MT, Honn KV, Nie D. Cyclooxygenases, prostanoids, and tumor progression. Cancer Metastasis Rev. 2007;26:525-534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 247] [Cited by in F6Publishing: 230] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 155. | Timoshenko AV, Chakraborty C, Wagner GF, Lala PK. COX-2-mediated stimulation of the lymphangiogenic factor VEGF-C in human breast cancer. Br J Cancer. 2006;94:1154-1163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 123] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 156. | Zhang XH, Huang DP, Guo GL, Chen GR, Zhang HX, Wan L, Chen SY. Coexpression of VEGF-C and COX-2 and its association with lymphangiogenesis in human breast cancer. BMC Cancer. 2008;8:4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 157. | Yoshinaka R, Shibata MA, Morimoto J, Tanigawa N, Otsuki Y. COX-2 inhibitor celecoxib suppresses tumor growth and lung metastasis of a murine mammary cancer. Anticancer Res. 2006;26:4245-4254. [PubMed] [Cited in This Article: ] |
| 158. | Tsujii M, DuBois RN. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell. 1995;83:493-501. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1569] [Cited by in F6Publishing: 1553] [Article Influence: 53.6] [Reference Citation Analysis (0)] |
| 159. | Basu GD, Pathangey LB, Tinder TL, Lagioia M, Gendler SJ, Mukherjee P. Cyclooxygenase-2 inhibitor induces apoptosis in breast cancer cells in an in vivo model of spontaneous metastatic breast cancer. Mol Cancer Res. 2004;2:632-642. [PubMed] [Cited in This Article: ] |
| 160. | Sugimoto T, Bartholomeusz C, Tari AM, Ueno NT. Adenovirus type 5 E1A-induced apoptosis in COX-2-overexpressing breast cancer cells. Breast Cancer Res. 2007;9:R41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 161. | Totzke G, Schulze-Osthoff K, Jänicke RU. Cyclooxygenase-2 (COX-2) inhibitors sensitize tumor cells specifically to death receptor-induced apoptosis independently of COX-2 inhibition. Oncogene. 2003;22:8021-8030. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 65] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 162. | Petersen C, Petersen S, Milas L, Lang FF, Tofilon PJ. Enhancement of intrinsic tumor cell radiosensitivity induced by a selective cyclooxygenase-2 inhibitor. Clin Cancer Res. 2000;6:2513-2520. [PubMed] [Cited in This Article: ] |
| 163. | Burd R, Choy H, Dicker A. Potential for inhibitors of cyclooxygenase-2 to enhance tumor radioresponse. In: RE Harris, ed. COX-2 Blockade in Cancer Prevention and Therapy. Humana Press, Totowa, NJ 2002; 301-311. [Cited in This Article: ] |
| 164. | Subbaramaiah K, Chung WJ, Dannenberg AJ. Ceramide regulates the transcription of cyclooxygenase-2. Evidence for involvement of extracellular signal-regulated kinase/c-Jun N-terminal kinase and p38 mitogen-activated protein kinase pathways. J Biol Chem. 1998;273:32943-32949. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 156] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 165. | Levitt RJ, Buckley J, Blouin MJ, Schaub B, Triche TJ, Pollak M. Growth inhibition of breast epithelial cells by celecoxib is associated with upregulation of insulin-like growth factor binding protein-3 expression. Biochem Biophys Res Commun. 2004;316:421-428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 166. | Subbaramaiah K, Norton L, Gerald W, Dannenberg AJ. Cyclooxygenase-2 is overexpressed in HER-2/neu-positive breast cancer: evidence for involvement of AP-1 and PEA3. J Biol Chem. 2002;277:18649-18657. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 245] [Cited by in F6Publishing: 253] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 167. | Benoit V, Relic B, Leval Xd Xd, Chariot A, Merville MP, Bours V. Regulation of HER-2 oncogene expression by cyclooxygenase-2 and prostaglandin E2. Oncogene. 2004;23:1631-1635. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 168. | Sivula A, Talvensaari-Mattila A, Lundin J, Joensuu H, Haglund C, Ristimäki A, Turpeenniemi-Hujanen T. Association of cyclooxygenase-2 and matrix metalloproteinase-2 expression in human breast cancer. Breast Cancer Res Treat. 2005;89:215-220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 169. | Larkins TL, Nowell M, Singh S, Sanford GL. Inhibition of cyclooxygenase-2 decreases breast cancer cell motility, invasion and matrix metalloproteinase expression. BMC Cancer. 2006;6:181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 124] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 170. | Kang JH, Song KH, Jeong KC, Kim S, Choi C, Lee CH, Oh SH. Involvement of Cox-2 in the metastatic potential of chemotherapy-resistant breast cancer cells. BMC Cancer. 2011;11:334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 171. | Singh B, Berry JA, Shoher A, Ramakrishnan V, Lucci A. COX-2 overexpression increases motility and invasion of breast cancer cells. Int J Oncol. 2005;26:1393-1399. [PubMed] [Cited in This Article: ] |
| 172. | Staveley-O’Carroll K, Sotomayor E, Montgomery J, Borrello I, Hwang L, Fein S, Pardoll D, Levitsky H. Induction of antigen-specific T cell anergy: An early event in the course of tumor progression. Proc Natl Acad Sci USA. 1998;95:1178-1183. [PubMed] [Cited in This Article: ] |
| 173. | Pockaj BA, Basu GD, Pathangey LB, Gray RJ, Hernandez JL, Gendler SJ, Mukherjee P. Reduced T-cell and dendritic cell function is related to cyclooxygenase-2 overexpression and prostaglandin E2 secretion in patients with breast cancer. Ann Surg Oncol. 2004;11:328-339. [PubMed] [Cited in This Article: ] |
| 174. | Hecht SS. Tobacco smoke carcinogens and breast cancer. Environ Mol Mutagen. 2002;39:119-126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 149] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 175. | Salaspuro M. Acetaldehyde as a common denominator and cumulative carcinogen in digestive tract cancers. Scand J Gastroenterol. 2009;44:912-925. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 176. | McGettigan P, Henry D. Cardiovascular risk with non-steroidal anti-inflammatory drugs: systematic review of population-based controlled observational studies. PLoS Med. 2011;8:e1001098. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 377] [Cited by in F6Publishing: 341] [Article Influence: 26.2] [Reference Citation Analysis (0)] |