Abstract
Objectives: To examine the changes in nitric oxide (NO), asymmetric dimethylarginine (ADMA), symmetric dimethylarginine (SDMA), and L-arginine levels in schizophrenia during acute psychotic exacerbation and in bipolar disorder during mania and to compare those changes to healthy controls.
Methods: Thirty schizophrenia patients with acute psychotic exacerbation and 30 bipolar disorder patients with mania, who attended the Psychiatry Department, Erenköy Hospital for Mental and Nervous Diseases, Istanbul, Turkey, in 2010. Thirty healthy controls were included. The diagnosis was made using the Structured Clinical Interview for Axis I Disorders (SCID-I) interviews. Patients’ demographic data were recorded, and NO, SDMA, L-arginine, and ADMA levels were studied.
Results: Nitric oxide levels in schizophrenia patients were significantly lower than the control group. Nitric oxide levels in the bipolar group were lower than the control group but the difference was not statistically significant. The levels of SDMA, ADMA, and L-arginine were found to be significantly higher in schizophrenia and bipolar disorder patients than the control group. The disease duration was slightly negatively correlated with NO levels in bipolar patients. In schizophrenia patients, the disease severity was slightly positively correlated with NO levels.
Conclusion: Significant changes in NO, SDMA, ADMA, and L-arginine levels in schizophrenia and bipolar disorder patients suggest that NO and inhibitors of NO might be implicated in the neurobiology of schizophrenia and bipolar disorder.
Nitric oxide (NO) is a paracrine signaling molecule in the regulation of endothelial vascular relaxation. Also, it can work as a neurotransmitter and, thus, plays a role in inflammatory and oxidant processes.1 Nitric oxide is a compound that has a quite short half-life, a lipophilic structure, and important antioxidative functions; NO synthase (NOS) is used for its synthesis from the amino acid L-arginine. It binds to hemoglobin in the body and may be effective wherever it is by transforming to nitrate and nitrite.2 Nitric oxide synthase is found in various parts of the body, and there are 3 known isoforms: inducible NOS (iNOS), endothelial NOS (eNOS), and neuronal NOS (nNOS).3
Natural L-arginine analogs such as L-N-monomethyl arginine (L-NMMA), asymmetric dimethyl-arginine (ADMA), and symmetric dimethyl-arginine (SDMA) may competitively inhibit NO synthesis.4 Asymmetric dimethyl-arginine is naturally found in cells and tissues, and it has free movement in the plasma. It is excreted in the urine and reduces the synthesis of NO by blocking the transport of L-arginine into cells and by NOS inhibition in high concentrations.5
Neuronal NOS also functions in the synthesis of NO in the CNS.6 Nitric oxide synthase is considered to be in an inactive state in the central nervous system (CNS).6 Nitric oxide, generated by neuronal NOS, has important roles in memory organization and the development of the brain. Some metal ions, calmodulin, and L-citrulline may influence the activation of neuronal NOS.1 Nitric oxide synthase becomes active as a result of the stimulation of neurotransmitters and neuromodulator systems such as glutamate, serotonin, acetylcholine, and noradrenaline, which are known to be associated with many neuropsychiatric diseases. When it is focused on the mechanisms involved in the synthesis and inhibition of NOS, their association with neuropsychiatric diseases has drawn attention. In recent years, the role of oxidative mechanisms has been investigated in relation to the pathophysiology of many psychiatric disorders, including schizophrenia (SCZ) and bipolar disorder in which neurotransmitters and amino compounds are also thought to play a significant role.2 Studies of these mechanisms have become increasingly frequent as they relate to NO and ADMA.7,8
Schizophrenia is a common, chronic, and devastating mental disease characterized by delusions, hallucinations, disorganized speech, and disorganized behavior.9 Studies have shown that SCZ has a worldwide prevalence of 0.3-0.7%.10
In bipolar disorder (BD), there are recurrent manic and depressive episodes. Bipolar disorder causes a severe decrease in functioning and an increase in mortality and morbidity.11 Although several models have been established for these diseases, their etiopathogenesis have not been clarified yet. For both SCZ and BD, an emerging body of evidence suggests that oxidative stress may be an important mediating factor in their etiology.12 Studies that revealed the role of NOS inhibitors in various physical and neuropsychiatric disorders have particularly focused on ADMA.13,14 On the other hand, the levels of SDMA, L-NMMA, and other NOS inhibitors have not been examined together in relation to NO and ADMA in BD and SCZ.
Although levels of NO and its natural inhibitor ADMA in NO metabolism have been studied before, no previous trial has assessed SDMA and L-arginine levels in SCZ and BD together. The changes in the balance of neurotransmitters (serotonin, dopamine, noradrenaline, and glutamate) take part both in the etiopathogenesis and composition of clinical symptoms.15 Such a balance, related to the activation of NOS, is known to play a role in these differences and thought to be associated with the neurobiological properties of both disorders.
As we mentioned above, according to the literature, NO, SDMA, ADMA, and L-arginine seem to have interactions with some neurotransmitters, which may be related to some mental illnesses, and they may also have roles on the neurobiology of mental disorders directly. The primary objective of the present study was to evaluate the role of NO and some NO synthesis inhibitors in BD and SCZ patients who experienced a symptomatic exacerbation. We particularly planned to include patients with SCZ during acute psychotic exacerbation and patients with BD during mania because we considered that exacerbation periods might reflect the pathology in the neurobiology of both diseases better than that during remission periods. These 2 diseases are of importance in psychiatry practice as well, and they may have a common neurobiological basis. We also aimed to investigate the correlation between clinical parameters such as disease duration and severity by evaluating the patient groups by clinical scales (young mania rating scale for BD group and brief psychiatric rating scale for SCZ group) and NO, L-arginine, ADMA, and SDMA levels. We hypothesized that NO, ADMA, L-arginine, and SDMA levels in SCZ patients during acute psychotic exacerbation and in bipolar patients during mania might be different from healthy controls, and the differences might reflect underlying biological processes during symptom exacerbation.
Methods
This was a cross-sectional case-control study. The age range of the included patients was between 18-65 years. Acute exacerbation of SCZ was diagnosed when the BPRS score was at or above 30, and the manic episode was diagnosed when the YMRS score was at or above 12 and the HDRS score was below 7. Patients who had any other concurrent psychiatric disorder as well as those who had a cardiovascular or cerebrovascular disease, diabetes mellitus, liver, or kidney dysfunction were excluded. Patients who were illiterate or had mental retardation were also excluded.
Fifty SCZ and 50 bipolar disorder patients were screened; 39 SCZ patients and 37 BD patients were accepted to participate in the study. Nine SCZ patients and 7 BD patients were excluded. In the final analysis there were 30 patients (14 males and 16 females) with SCZ and 30 patients (14 males, and 16 females) with a manic episode of BD according to DSM-IV-TR criteria.
Patients included in the study were recruited among the patients who attended to the Psychiatry Department Erenköy Hospital for Mental and Nervous Diseases, Istanbul, Turkey, in 2010. Approval for the study was taken from Firat University Ethics Committee. Thirty healthy volunteers (12 males and 18 females) who did not have any current complaints or known diseases served as control subjects. None of the participants had a history of drug abuse. Some were social alcohol drinkers or smokers.
Information about the study was given to all participants, and verbal and written informed consent was obtained from each of them. The study was conducted in accordance with the principles of the Declaration of Helsinki. Sociodemographic data of the groups are shown in Table 1.
Sociodemographic features of the participants (n=30).
Clinical assessment scales
Structured clinical Interview (SCID-I) for DSM-IV Axis I disorders: the aim of this scale was to make diagnoses according to DSM-III-R criteria. The scale was later updated for DSM-IV.16 The validity and reliability of the Turkish version of the scale were studied.17
Young mania rating scale (YMRS): this scale was developed to measure the severity of the manic episode symptoms in BD.18 The validity and reliability of the scale in Turkish have been demonstrated.19 Patients with acute mania whose score less than 12 points are considered as being in remission.
Hamilton depression rating scale (HDRS): this scale measures the level and change of the severity of depression in patients. The scores less than 7 suggest the absence of clinical depression.20 The validity and reliability of the scale in Turkish have been shown.21
Brief psychiatric rating scale (BPRS): this scale was developed to measure the severity and change in the severity of psychotic and depressive symptoms in SCZ and other psychotic disorders. A score of 30 or more represents a major syndrome.22 The validity and reliability of the scale in Turkish have been proven.23
Biochemical measurements
Blood samples were drawn in the morning on an empty stomach and collected into anticoagulant-free vacuum tubes. After clotting, the samples were centrifuged at 3000 rpm for 10 minutes. Serum aliquots were stored at -80°C until they were assayed and thawed immediately prior to the measurement of the biochemical parameters. Biochemical parameters were measured using an autoanalyzer (Abbot Brand kits, Abbott Corp., USA) (Abbott Architect C 8200 I, Abbott Corp., USA).
Serum total NO measurement
There are several methods to measure serum total NO levels. Most of the assays use colorimetric methods based on direct nitration or with organic compound oxidation of the nitrate.24,25 Because of the limitations of these methods, some more sensitive but also quite expensive methods, such as enzymatic or ion chromatography, were developed.26,27 Cortas and Wakid’s method, which we used in our study, is a sensitive and cheap method that does not require much equipment.28 In this method, first, the reduction of nitrate to nitrite by metal than diazotization and the measurement of the level of nitrite were used. Total NO levels were measured by measuring the levels of its durable metabolites nitrite (NO2) and nitrate (NO3) because NO produced in biological systems is oxidized to NO2 and NO3 rapidly such as in 2-30 seconds. Measuring NO2+NO3 levels is a common method for measuring NO levels.29
Serum arginine, SDMA and ADMA measurement
L-arginine levels and its methylated derivatives ADMA and SDMA were measured by using a EUREKA (Headquarters: Via E. Fermi 25 60033 Chiaravalle (AN) ITALY) fluorescence detector assay with high-performance liquid chromatography (HLPC) method.30
Statistical analysis
Data obtained in the study was shown as mean±standard deviation (SD). The variables evaluated in this study were normally distributed (checked with Kolmogorov-Smirnov test and histogram), and the number of cases was deemed adequate for the application of the parametric tests. The one-way analysis of variance test (ANOVA) and post hoc LSD and Tukey B tests were performed for binary comparisons between the groups. The chi-square test and Fisher-Freeman-Halton test were used for the comparison of categorical variables. A p<0.05 was considered statistically significant.
Power analysis
A post hoc power analysis was performed after the study by using the data for the ADMA variable with G*Power 3.1.9.4 for Windows (Open Source) package program. The power of the study was found to be 95.7%.
Results
When comparing the biochemical parameters of the study groups, there were no significant differences in blood glucose, urea, or creatinine between the controls and BD patients (Table 2). When comparing the lipid parameters, no significant differences were observed in total cholesterol, HDL, and LDL levels between the controls and BD patients. Triglyceride levels were significantly higher in the BD group compared to the controls (p<0.004) (Table 2).
Comparison of several biochemical parameters among the groups (N=30).
When the control group and SCZ patient group were compared for biochemical parameters, no significant difference was observed in blood glucose, urea, or creatinine levels (Table 2).
Although the levels of total cholesterol in lipid parameters were higher in the SCZ patient group than in the controls, this increase was not statistically significant. A statistically significant difference was observed in HDL cholesterol levels between the controls and SCZ patient group (p<0.009) (Table 2).
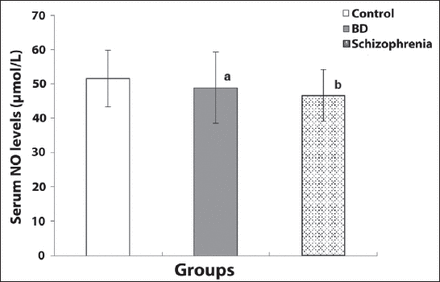
Nitric oxide levels were observed to be 46.63±7.52 µmol/L in the SCZ patient group, which was significantly lower than for the controls (51.59±7.84 µmol/L) (p<0.05) (Table 2). Although lower NO levels were observed in the BD patient group (48.87±10.37 µmol/L) compared to the controls (51.58±8.3 µmol/L), this difference was not statistically significant (Table 2).
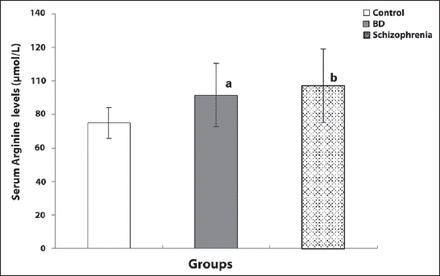
The SCZ patient group had statistically significant higher levels of serum L-arginine (97.12±21.92 µmol/L) than the control group (74.75±9.19 µmol/L) (p<0.001) (Table 2). The BD patient group also had statistically significant higher levels of serum L-arginine (91.57±18.84µmol/L) than the controls (74.75±9.19 µmol/L) (p<0.001) (Table 2).
In a similar manner, as with L-arginine, the serum levels of ADMA were observed to be significantly increased in SCZ patients (12.12±3.01 µmol/L) compared to the controls (5.72±1.81 µmol/L) (p<0.001) (Table 2). Also, serum ADMA levels were observed to be significantly increased in the BD patient group (10.77±3.73 µmol/L) compared to the controls (5.72±1.81) (p<0.001) (Table 2).
While the mean SDMA level was 4.87±0.81 µmol/L in the SCZ patient group it was 3.67±0.98 µmol/L in the controls. Symmetric dimethyl-arginine level was observed to be significantly higher in the SCZ patient group compared to the controls (p<0.001) (Table 2). Serum SDMA level was also observed to be significantly higher in the BD patient group (4.52±0.85 µmol/L) compared to the controls (3.67±0.98) (p<0.001) (Table 2).
According to the correlation analyses for patients with BD, disease duration was slightly significantly negatively correlated with NO levels (r= -0.384, p=0.036; Figure 1). Disease severity was not significantly correlated with NO, ADMA, SDMA, and L-Arginine levels in the BD group. In patients with SCZ, only disease severity evaluated with BPRS was slightly significantly positively correlated with NO levels (r= 0.376, p=0.037) (Figure 2). Disease duration was not significantly correlated with NO, ADMA, SDMA, and L-Arginine levels in the SCZ group.
Scatter plot for the association between nitric oxide level and disease severity in schizophrenia patients.
Scatter plot for the association between nitric oxide and disease duration in bipolar patients.
Discussion
In this study, we found that the levels of ADMA, SDMA, and L-arginine were the highest in the SCZ group and the lowest in the controls, with the BD group’s values in-between. Also, a weak negative correlation between NO levels and disease duration for the BD group and a weak positive correlation between NO levels and disease severity for the SCZ group were detected.
NO levels
A previous study demonstrated a decrease in the levels of NO in patients, including first-episode SCZ patients, compared to healthy controls. This finding was interpreted as a reduction in the NO system in SCZ. In another study, investigating patients who have been drug-naive for at least 4 weeks irrespective of disease severity, exacerbation, remission, etc. serum levels of NO were found to be lower in all subtypes of SCZ and especially more pronounced in the paranoid subtype compared to the controls. Also, the age of onset, the duration of illness, schizophrenic subtype, disease severity, or the dose of antipsychotic drugs used for treatment did not affect the levels of NO in pre and 6-week post-treatment periods. It was suggested that NO could be an important molecule in explaining the etiopathogenesis of SCZ.31 Our results are in agreement with most of the studies reported in the literature.31 In contrast to our findings, a study reported that NO levels were increased in SCZ patients compared to those in the controls.32 The reason for this can be considered to result from the complexities of the etiologies of SCZ and the different measurement methods of NO. The heterogeneity of the symptoms, disease subtypes, whether being in remission or exacerbation of psychotic symptoms for patients, might have affected the results. Similar mechanisms causing the disease may eventually differ among patients and the subtypes of the diseases are not fully known yet. Nitric oxide acts as both an antioxidant and prooxidant; therefore, it is unclear whether the change in NO levels is a causal, resulting, or compensating mechanism of these diseases and many mechanisms other than NO system have an effect on the biology of diseases.32,33
Furthermore, the mean value of plasma NO levels in the BD patients with a manic episode was found as significantly higher than that of controls in one study.33,34 Moreover, NO acts as a retrograde transmitter in glutamatergic synapsis and as a type of glutamate receptor. N-methyl D-aspartate receptors play an important role in the neurobiology of depression and the medications of NMDA receptor antagonists; and lithium has been proven to have antidepressant effects by increasing NO levels.8,34 Conflicting results from the studies of NO levels and mood disorders may be associated with the methodological differences and the complex etiopathological causes and various clinical features of BD. According to these data, we suppose that the effect of NO should be explored in BD during depressed, manic, mixed episodes as well as in remission.
Our results suggesting lower but statistically not significant NO levels in the BD mania group than those in the controls, and a weak negative correlation between disease duration and NO levels might have resulted from the heterogeneity of the disease and from being in a manic episode not a depressive or a mixed episode for patients as well. According to one study, depression might be more related to lower NO levels than mania might.7
Consequently, NO, a free radical which can work as an antioxidant, and inhibitors of NO might be related to many neuropsychiatric diseases and NO related mechanisms may play a role in the etiology of such psychiatric disorders as SCZ and BD, in which cognitive pathologies like attention and memory deficits perceptional pathologies like hallucinations, and physiological pathologies like appetite, all of which are associated with abnormalities in neurotransmitter systems mentioned above are seen.12
Levels of ADMA, SDMA, and L-Arginine
Studies with NO synthesis inhibitors have focused principally on ADMA. While NO levels fell, ADMA levels were found to be higher in patients with Alzheimer’s disease and major depression compared to controls.7,8 Asymmetric dimethyl-arginine may cause a reduction in cerebral blood flow by inhibiting NO, reducing pliability in the vascular structure, and increasing vascular resistance. Asymmetric dimethyl-arginine may also play a role in cognitive symptoms through acetylcholine associated with frontal lobe hypometabolism in different brain regions by affecting the brain blood flow in SCZ.35,36 Further study of patient subgroups with other cognitive deficits and negative symptoms may provide guidance on this issue. In a recent study including the first episode SCZ patients, it was detected that ADMA levels were higher than those in the controls, and the levels returned to normal after 2 months of antipsychotic therapy but ADMA levels were not correlated with clinical severity scores.37
In our study, the levels of ADMA, L-arginine, and SDMA, which are NO inhibitors of increasing strength, respectively (even though there was no statistically significant difference between the SCZ and BD patient groups) suggested that they may be important in relation to the psychosis spectrum and the severity of psychosis in these diseases. Our results also showed that there might be impaired antioxidant mechanisms in SCZ and BD. Schizophrenia is at one end of the psychosis spectrum while disorders such as BD, short psychosis, and psychotic depression, are near the other end.38 Moreover, over-release of NO and decrease in the levels of NO depend on the structure of the antioxidant and possible glutamatergic neurotoxicity, and such a dependence can be considered to be associated with glutamatergic pathology in the neurobiology of SCZ and BD.39
No significant correlation was found between both SCZ and BD mania in terms of disease severity and duration, ADMA, SDMA, and L-arginine levels, which might have resulted from a small sample size of the study, heterogeneity of the diseases, or a limited association between ADMA, SDMA, and L-arginine levels and both diseases.
One of the strengths of the current study was the exclusion of individuals with chronic medical illnesses. We encourage future studies of the parameters examined here with larger sample sizes and the inclusion of patients experiencing recurrent disease episodes. Such studies could provide more detailed information on this issue.
Study limitations
The small sample size was one of the limitations of this study. Schizophrenia is an ongoing disease with acute exacerbations and remissions. Bipolar disorder is a disease characterized by periods of manic, mixed symptoms, depression, and remission. The patients in this study were selected during acute psychotic exacerbation of SCZ and acute manic episodes of BD. Thus, a limitation of this study was that patients in remission and other episodes of these diseases were not examined. All of the patients in both SCZ and BD groups were under drug therapy such as antipsychotics, mood stabilizers and benzodiazepines. None of the effects of medications was assessed in this study. Moreover, we had no data with respect to the burden of the disease and the number of episodes. Another limitation is about statistical analysis. We performed binary comparisons of the 3 groups for NO although ANOVA among the 3 groups was not significant because one of our hypotheses was a change in NO level in SCZ patients compared to that in controls.
In conclusion, the levels of NO were found to be lower, and ADMA, SDMA, and L-arginine were found to be higher in patients with SCZ and BD compared to controls in our study. These findings are generally consistent with the literature.7,30,31 Moreover, increasing levels of ADMA, L-arginine, and SDMA in the neurobiology of both disorders may lead to a decline by affecting NO levels. From this perspective, the primary pathology in both disorders may be associated with the increases in NO inhibitor levels, and the secondary pathology may be associated with the reductions in NO. All these data suggest that NO and its inhibitors may be important in the neurobiology of SCZ and BD.
Cumulative data on oxidant-antioxidant systems and their roles on neuropsychiatric diseases may enable improvements in new treatment strategies that would include NOS inhibitors in the treatment of SCZ and BD.
We urge long-term follow-up studies that might reveal further changes in the parameters we measured here. Such studies would be especially valuable for the first-episode patients of both diseases who do not use drugs. Ideally, these studies should investigate different stages of the diseases and consist of larger study groups to examine the clinical, neuroimaging, neuropsychological, and electrophysiological aspects of this phenomenon.
Acknowledgment
The authors gratefully acknowledge Model Statistical Modelling Center for English language editing.
Footnotes
Disclosure. Authors have no conflict of interests, and the work was not supported or funded by any drug company.
- Received July 30, 2019.
- Accepted December 4, 2019.
- Copyright: © Saudi Medical Journal
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.