ABSTRACT
Objectives: To discuss our experience with managing cochlear implant cases that required revision surgery.
Methods: A retrospective case series study including data from 922 cochlear implant patients at an academic tertiary center was evaluated retrospectively. All patients who underwent revision cochlear implant (CI) surgery between January 2011 and July 2017 were included. The following data were collected: patient demographic data, details on the first implant, reasons for the revision, duration from initial implantation to revision, type of device, and management.
Results: Out of 922 CI patients, 37 (4%) underwent revision surgery, comprising 33 children and 4 adults. The most common reason for revision surgery, at 28/37 cases (75.6%), was device failure. Surgical and medical aetiologies were responsible for 9/37 (24.3%) revisions. The mean duration from the initial implantation to the revision surgery was 29 months.
Conclusion: Revision CI surgery is not uncommon after initial implantation. Cochlear implant programs must implement long-term follow-up processes for CI users. Whenever a patient’s rehabilitated performance regresses, the cause should be investigated to determine whether subsequent reimplantation is necessary.
In the last 3 decades, cochlear implants (CIs) have been used as an effective rehabilitation approach for individuals with severe to profound sensorineural hearing loss. The expansion of cochlear implantation throughout the world has increased the number of complications being reported. Certain complications, such as device failure, infection refractory to medical treatment, flap necrosis, and migration of the receiver or the electrode that affects the auditory outcome, are indications for revision surgery.1 Several published studies have shown variable rates of CI revision. The first reported revision surgery was performed by Hochmair-Desoyer and Burian2 in 1985. The indications for revision are numerous; many studies report that device failure is the most common complication that requires revision surgery.3 A revision is considered successful when the CI recipient derives a functional benefit from reimplantation.4
This article presents the authors’ experience with revision CI surgeries at tertiary Ear Center, one of the largest institutes in the Middle East. We aimed to discuss our experience with managing CI cases that required revision surgery. Moreover, a part from previous reports we propose a management diagram for cases of suspected device failure.
Methods
This is a longitudinal a retrospective cohort study of 922 CIs performed between January 2011 and July 2017 at tertiary ear center. All patients who underwent revision CI surgery with or without reimplantation were included. Adult and pediatric patients (<18 years at time of implantation) were included in this study. We excluded patients of incomplete file and who had complication post CI managed without surgical intervention. All implants were performed using the standard surgical approach of mastoidectomy, access through a facial recess, and placement of the receiver-stimulator under the subperiosteal pocket in a drilled-out bony well without suture fixation. In cases of revision, we try as much as we can to remove the implant intact as one piece for cases need to be implanted; on the second stage we cut the electrode at the level of facial recess.
Data regarding patient demographic, details of the first implant, reasons for the revision, duration from initial implantation to revision, data of CI manufacture, and management were collected.
The data were tabulated and analyzed using the Excel program. Based on the review of the present cases, our multidisciplinary team from audiology, speech and otolaryngology units proposed a diagram for managing the cases of suspected device failure. A literature review was also performed. The study protocol was approved by the ethical committee of the University Institutional Review Board.
Results
A total of 922 CIs were performed during the above mentioned period (n=820 [88.9%] were pediatrics, and n=102 [11%] were adults). Thirty-seven patients (4%) underwent revision surgery at our center, including 21 (56.7%) males and 16 (43.2%) females. The pediatric group comprised 33/37 (89%) patients with a mean age of 30 months, and the adult group comprised 4/37 (10.8%) patients with a mean age of 43.6 years. The rate of revision in the pediatric group was 33/820 (4%), whereas in the adult group, it was 4/102 (3.9%). The mean duration of device usage from first implant to revision was 29 months (range one day - 73 months).
The most common reason for revision surgery was device failure 28/37 (75.6%). The device failure was reported more in pediatric age group, where 27 out of 28 device failure patients were children and only one adult case. The rate of device failure in the pediatric group was 27/820 (3.3%), whereas in the adult group was 1/102 (1%). The mean duration of device usage before device failure was 25.5 months (range from 6 weeks to 64 months).
An analysis of the type of implants that required revision due to device failure (Table 1) revealed that 20/693 (2.9%) devices from MedEl Corporation (Innsbruck, Austria) and 8/200 (4%) devices from Cochlear Corporation (Sydney, Australia) experienced failure.
- Summary of revision cases based on implant companies.
A review of the final manufacturing analysis report provided by the device companies showed that each company had a certain sequence that could affect its devices. Fifteen out of 20 implants manufactured by the Med-El Corporation failed due to external mechanical impact, whereas 5/20 failed due to micromovements that led to electrode fatigue. Two out of 8 implants manufactured by the Cochlear Corporation experienced device failure due to external mechanical impact, whereas 6 implants failed due to leakage in the CI512 serial implant, which was recalled from the market later due to hermeticity issues in the receiver.
A total of 17 out of 28 devices were damaged by the external mechanical impact based on the manufacture report, only 5 of them had positive history for mechanical trauma. All failed devices were simultaneously explanted and replaced with a new one.
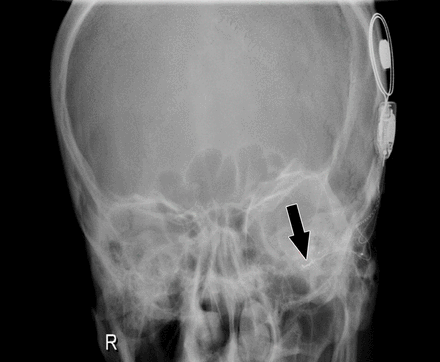
The present study showed that surgical and medical aetiologies were responsible for 9 of 37 revisions (24.3%). Among all the revision cases, 4 (10.8%) were due to infection. Trial of medical management including intravenous antibiotics with extensive local wound care, was unsuccessful. Therefore, all those 4 patients underwent staged revision surgery. Two patients (5.4%) had an electrode that was misplaced in the hypotympanum (Figure 1).
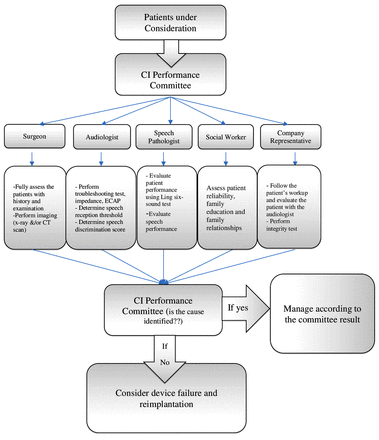
- Diagram showing the management of cases with decreased performance after cochlear implant surgery.
An extensive iatrogenic cholesteatoma after a trans-canal CI performed outside our center was observed in one patient. One patient (2.7%) who received a CI for single-sided deafness was not satisfied after cochlear implantation and experienced discomfort from the device. He complained of uncontrollable pain, insisted on removing the device and refused to be reimplanted. One patient (2.7%) required surgery for magnet reposition due to magnet dislocation after head trauma.
The operative procedures for the revision surgery was carried out either as one stage or 2 stages based on the reason of the revision. The cholesteaoma and flap infection cases underwent 2 stages surgery, where in the first surgery (ex-plantation) the device was removed and the electrode left inside the cochlea by cutting it at the level of facial recess. The second operation (re-implantation) often takes place 6 months later. The other remaining cases underwent one stage surgery.
Successful reimplantations were performed in 36 patients (97.3%), whereas no reimplantation occurred in one patient.
Discussion
In the literature, the rate of implants required revision surgery was reported to be between 3.6% and 11%.4-8 In the current study, 4% of total cochlear implanted patients underwent revision surgery. The most common reason for revision was device failure.3 We found that pediatric users of the CI had more risk of device failure than adults. The chance of accidental head trauma in playing, and risk of falling are more in the pediatric age group. Moreover, some of the children had neglected history of head trauma where the family thought is not significant.
Device failure can be suspected when the patient stops benefitting from the implant or experiences decreases in performance, but these factors do not necessarily indicate a device failure. At our center, we are following a protocol in cases with decreased performance (Figure 2). The importance of this diagram is to increase the awareness how to pick up the cases early and to manage them. These patients should be discussed by a review committee (CI performance committee) comprising a surgeon, audiologist, speech pathologist, social worker and one person representing the manufacturer of the device. The CI performance committee reviews the clinical and social picture of the patient to determine the cause of the decrease in performance. If no significant reasons were found, the suspicion of soft failure emerges. Soft failure is a term used when there is a decline in subject performance with no detectable issue in routine hardware tests. Patients or parents need to be counselled well regarding the committee’s findings as well as all management options and the risks and benefits of reimplantation.
- Postoperative x-ray showing a left cochlear implant electrode misplaced in the hypotympanum (arrow).
Only 10.8% of revision cases were because of infection and subsequently led to flap necrosis and device extrusion. In the literature, the incidence of infection-related revision in subjects with a CI ranges from 1.6% to 15%.9,10 We found that device extrusion was the second most common cause of revision after device failure. However, it is known that not all extrusion cases are due to infection. Silicon allergy, although rare, can cause extrusion and is difficult to distinguish from infection.4
Numerous cases of electrode misplacement are described in the literature, and such cases reportedly account for between 6% and 10% of revision cases.7,8 Certain situations can result in electrode misplacement, such as cochlear malformation, abnormal middle ear landmarks and the surgeon’s experience.11 The surgeon can avoid this complication by following anatomical landmarks before looking for the round window, which includes the pyramid and stapedial tendon. Furthermore, radiological imaging can be used intraoperatively to evaluate the position of the electrode whenever the surgeon is in doubt.
The reported prevalence of cholesteatoma among post CI major complications varies in the literature; Brito et al12 reported 6/49 (12.2%) after a mean implantation time of 45±13 months. Cochlear implant patients require long-term follow-up for the detection of such late complications, particularly in the presence of an external ear canal defect or annular injury.12
In the present study, one patient experienced discomfort from the device. Therefore, he underwent explantation. Shapira et al13 reported a case series of patients in which pain over the receiver was described as a mild delayed complication in 2.8% of 1044 implants. Magnet reposition due to magnet dislocation resulting from head trauma, was reported in the current study. Hassepass et al14 reported that the most common cause of magnet dislocation is MRI accounting for 1.23%, followed by head trauma (0.17%) of 1076 implants. In revision cases, difficulties arising from neo-osteogenesis, fibrosis, and granulation are expected.15 Revision surgery is very safe, and full-electrode insertion can be achieved in most cases with manageable difficulties. The study’s limitation needs to compare more different CI manufacture to evaluate more for devices reliability. A systemic review of the same objective is needed for more powerful evaluation.
In conclusion, revision CI surgery is not uncommon, particularly with the increasing number of CI users. Cochlear implant programs must implement long-term follow-up processes for CI users because a variety of complications can occur at any time. Decline in patients performance needs a multidisciplinary approach as suggested in the current study. Companies need to work more to limit the instances of device failure.
Withdrawal policy
By submission, the author grants the journal right of first publication. Therefore, the journal discourages unethical withdrawal of manuscript from the publication process after peer review. The corresponding author should send a formal request signed by all co-authors stating the reason for withdrawing the manuscript. Withdrawal of manuscript is only considered valid when the editor accepts, or approves the reason to withdraw the manuscript from publication. Subsequently, the author must receive a confirmation from the editorial office. Only at that stage, authors are free to submit the manuscript elsewhere.
No response from the authors to all journal communication after review and acceptance is also considered unethical withdrawal. Withdrawn manuscripts noted to have already been submitted or published in another journal will be subjected to sanctions in accordance with the journal policy. The journal will take disciplinary measures for unacceptable withdrawal of manuscripts. An embargo of 5 years will be enforced for the author and their co-authors, and their institute will be notified of this action.
Acknowledgment.
The authors gratefully acknowledge the Saudi Society of Otolaryngology for sharing topics needed to be evaluated. Also the authors would like to thank AJE for English language editing.
Footnotes
Disclosure. Authors have no conflict of interests, and the work was not supported or funded by any drug company.
- Received August 24, 2020.
- Accepted December 16, 2020.
- Copyright: © Saudi Medical Journal
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial License (CC BY-NC), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.