Abstract
Objectives: To compare the primary patency and restenosis rates in treatment naieve dialysis arteriovenous fistulas (AVFs) after drug-coated balloons (DCB) versus plain balloon angioplasty (PTA).
Methods: This retrospective study included 157 patients who underwent AVF angioplasty for treatment-native AVF stenosis between January 2012 to 2022. The fistulas were Brachiocephalic (75%), Brachiobasilic (17%), and radiocephalic (8%). The index intervention was with either DCB or percutaneous transluminal angioplasty (PTA) with subsequent follow up. Patients with central venous stenosis, thrombosed fistula, fistula stents, AV graft or surgical intervention after the index procedure were excluded.
Results: Arteriovenous fistula angioplasty was done in 28 patients using DCB and in 129 patients using PTA. A total of 108 patients presented with a single stenosis, 42 with 2 stenoses, and 7 with 3 stenoses. The location of these stenoses was in the venous outflow (57%), the juxta anastomotic segment (31%), and cephalic arch (12%). The median time to re-intervention for the PTA was 216 days compared to 304 days for the DCB (p=0.079). Primary patency at 6 months was 60.4% for PTA and 75% for DCB (p=0.141)
Conclusion: Although DCB angioplasty of treatmentnaïve dysfunctional AVF tends to improve the time to intervention and 6-month primary patency compared to PTA, this difference did not reach statistical significance.
Arteriovenous fistula (AVF) is the dialysis access of choice. However, stenosis remains a major concern resulting in repeated endovascular procedures and may ultimately result in access failure. While balloon angioplasty is an accepted treatment for AVF stenosis, access dysfunction rates remain high due to restenosis.
Drug-coated balloon (DCB) technology is proven effective in minimizing neointimal hyperplasia in peripheral arterial disease supported by clinical trials.1
The use of DCBs in dysfunctional AVF remains controversial.2-9 Several studies indicated that DCB results in longer primary circuit patency and time to re-intervention with fewer interventions per patient.6,7,9 However, other trials and a meta-analysis found no statistically significant difference in target lesion primary patency at 3, 6, 9, and 12 months.2-5,10,11 This 10-year single center study aims to retrospectively evaluate the use of DCBs as a primary treatment for treatment naïve dysfunctional AVFs compared to standard percutaneous transluminal angioplasty (PTA), and tries to determine the predictors that may influence the patency rates.
Methods
This retrospective study was approved by the institutional review board and informed consent from patients was waived. This study aims to compare the time to re-intervention and primary patency of treatment-naïve dysfunctional dialysis AVFs after drug-coated balloons or plain balloon angioplasty. Primary patency is defined as the interval from the first index intervention following hemodialysis initiation via a functional AVF to the subsequent intervention to maintain the AVF function. The radiology information system data inquiry yielded a total of 1779 AVF procedures performed between Jan 2012 to Jan 2022 at King Abdulaziz Medical City, Riyadh. The study included 157 patients after applying the inclusion and exclusion criteria. The inclusion criteria are patients who are >18 years and have an index intervention with either DCB or PTA with subsequent follow up intervention. The exclusion criteria are patients with any prior fistula intervention, central venous stenosis, thrombosed fistula, preexisting fistula stents, AV graft or those who had a surgical intervention after the first angioplasty. For PTA, a variety of brands were used including Mustang and Sterling (Boston scientific, MA, USA), Dorado and Conquest (BD, AZ, USA), Passeo (Biotronik Inc, OR, USA). Dru-coated balloons included the In-PACT (Medtronic, MN, USA) with 3.5 μg/mm2 Paclitaxel drug dose and Lutonix (BD) with 2 μg/mm2 Paclitaxel drug dose. Data were collected on REDCap (REDCap, V11, Vanderbilt University, USA) pre-approved data collection forms and analyzed using the Statistical Package for the Social Sciences, version 26.0 (IBMCorp, Armonk, NY, USA). Categorical data were presented as count and percentages and numerical data were presented as mean and standard deviation. Mann-Whitney test was used to asses time to re-intervention by type of balloon. The Chi-square test was used to assess the association between time to re-intervention (within 6 months versus more than 6 months) and other categorical variables as well as to assess the association between stenosis location and the severity. A test was considered statistically significant of p-value of <0.05.
Results
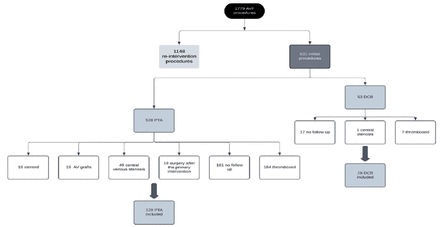
There were 1779 AVF procedures performed between Jan 2012 to 2022, 1148 of them were re-intervention procedures, and the remaining 631 were initial procedures. Out of the 631 initial procedures, 578 were PTA, of which 184 were thrombosed fistulas, 19 had surgery after the primary intervention, 49 had central venous stenosis, 18 were AV grafts, 18 had stent placement during the index procedure, and 161 had no follow up; thus 129 were included for the PTA group. There were 53 DCB primary interventions, 7 of them were thrombosed, 1 had central stenosis, and 17 had no follow up; thus 28 were included in the study; 4 of them had no follow angioplasty due to kidney transplant. The date of transplant was marked as the follow up date (Figure 1).
- Study population selection flow diagram. AVF: arteriovenous fistula, DCB: drug-coated balloons, PTA: plain balloon angioplasty
The mean age was 68 years + 15 (ranging between 23-99) and male patients were 39% while female were 61%. The majority of fistulas were brachiocephalic (75%), followed by brachiobasilic (17%) and radiocephalic (8%). The mean time to re-intervention for the entire cohort was 424 (ranging between 11-1934 days) days.
The location of these stenoses was mostly in the venous outflow 121 (57%) lesions followed by the juxta anastomotic segment 66 (31%) lesions and cephalic arch 26 (12%) lesions. The juxta anastomotic segment had 14 mild stenoses (21%), 24 (36%) moderate stenoses and 28 severe stenoses (43%). The venous outflow segment had 18 mild stenoses (15%), 49 (40%) moderate stenoses and 54 (45%) severe stenoses. The cephalic arch segment had 7 (27%) mild stenoses, 14 moderate stenoses (54%) and 5 (19%) severe stenoses. The comparison between these groups did not yield a statistically significant result (p=0.125).
The mean time to re-intervention for the PTA group was 408 days with a median of 216 days; while for the DCB group, the mean was 485 days with a median of 304 days (p=0.079). Primary patency at 6 months was 60.4% for the PTA group and 75% for the DCB group (p=0.141) (Table 1).
- Drug-coated balloons versus conventional percutaneous transluminal angioplasty time to re-intervention.
Time to re-intervention did not significantly differ by neither the severity of stenosis (p=0.455), nor the multiplicity of lesions (p=0.889) or the type of fistula (p=0.955) (Table 2).
- Predictors for time to re-intervention within 6 months or more for the entire cohort.
Discussion
Hemodialysis access stenosis is multifactorial and mainly caused by neointimal hyperplasia (NIH), and repeated angioplasty results in scarring from repeated trauma to the treated vessel.12,13 The use of drug-coated balloons in the management of dysfunctional dialysis fistulas has recently gained increasing attention to counteract the inflammatory response induced by angioplasty and to minimize the restenosis rates and number of re-interventions. Several recent studies have shown short term benefit of DCBs with improved 6-month patency rates and reduction of the time to re-interventions. Lookstein et al6 conducted a single blinded multi-institutional randomized clinical trial comparing the In-PACT AV DCB (Medtronic) to PTA in fistulas with new or recurrent stenotic lesions in 330 patients. The 6-month target lesion primary patency (TLPP) rate was superior with DCBs (82.2%) compared to PTA (59.5%) with similar safety profile.6 This difference was sustained at 12-month follow up and the TLPP was 63.8% in the DCB group compared with 43.6% in the PTA group (p<0.001). The use of DCBs was associated with a 35.4% reduction in re-interventions to maintain TLPP at 12 months (93 in the DCB group and 144 in the PTA group).9 Trerotola et al7 evaluated the Lutonix DCB in a randomized trial on 285 patients and found no significant difference in TLPP at 6 months (71% ± 4% for DCB and 63% ± 4% for PTA, p=0.06). However, DCB was associated with significantly fewer interventions to maintain patency (DCB 0.31 versus PTA 0.44 intervention per patient; p=0.03) with similar safety profiles.7 The PAVE trial compared Lutonix DCBs to high-pressure PTA in 212 patients and showed no difference in the time to target lesion restenosis or patency related outcomes.14 Another single center-single-blinded RCT of 42 patients who underwent angioplasty with either Advance 18 DCB (Cook Medical, Bloomington, IN, USA) or Advance PTA balloon. The trial showed no significant differences in number of interventions or freedom from target lesion restenosis at 12 months.8 A recent meta-analysis of 15 RCTs included a total of 1535 patients and showed no significant differences of TLPP rates at 3, 6, 9, and 12 months between DCBs and PTA.11 The current study presents a real-life retrospective comparative evaluation of the time-to-intervention in treatment naïve fistulas after excluding all fistulas that had prior treatments. Prior or repeated angioplasties might increase the severity of vessel trauma and subsequent neointimal hyperplasia dampening the effect of angioplasty on the vessel wall. Drug interactions with intima and vascular smooth muscles may change with repeated wall trauma, which could hinder its effect on subsequent delivery compared to the primary intervention.
In conlusion, this study is limited by the low number of DCBs. Although not statistically significant, the difference in 6 months primary patency and time to re-intervention trends in favor of DCBs compared to PTA. This is in line with several prior trials, which have included patients with both treatment naive and restenotic lesions.
Further subgroup analysis of patients in prior trials based on the number of previous angioplasty procedure may help identify the impact of paclitaxel as a primary treatment rather than a bailout or secondary intervention.
Acknowledgment
The authors would like to acknowledge King Abdullah International Medical Research Center Publication office for the English language editing.
Footnotes
Disclosure. Authors have no conflict of interests, and the work was not supported or funded by any drug company.
- Received November 27, 2023.
- Accepted May 16, 2024.
- Copyright: © Saudi Medical Journal
This is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.