Abstract
Objectives: To analyze the efficacy and adverse events (AEs) of pirfenidone in idiopathic pulmonary fibrosis (IPF) trials.
Methods: MEDLINE, Cochrane Library, and ClinicalTrials.gov were searched for studies published before June 2016. All studies of clinical trials with the key words IPF or idiopathic pulmonary fibrosis or lung fibrosis and pirfenidone or Esbriet were identified. Quality assessment and data extraction were conducted by 2 independent researchers. A meta-analysis of randomized controlled trials (RCTs) was performed, and relative risk (RR) and 95% confidence intervals (95% CIs) were calculated.
Results: Five studies were included in this review, involving 1568 participants. The meta-analysis revealed that pirfenidone reduced the risk of decline in forced vital capacity (FVC)% ≥10% from baseline (relative risk: 0.62; 95% CI: 0.51-0.76, p<0.001). The pirfenidone group had a significantly higher rate of AEs compared with the placebo group. Pirfenidone did not reduce mortality from any cause significantly (odds ratio: 0.63; 95% CI: 0.36-1.09).
Conclusions: This study showed that pirfenidone could reduce disease progression as assessed by the decline in FVC in IPF. Pirfenidone represents a suitable treatment option for patients with IPF.
Idiopathic pulmonary fibrosis (IPF) is an irreversible and ultimately fatal chronic fibrotic lung disease associated with dyspnea and a progressive decline in lung function. Idiopathic pulmonary fibrosis is the most frequent and severe entity of idiopathic interstitial pneumonias,1 with a median survival from the onset of symptoms of only 2.8-4.2 years. The incidence and prevalence of IPF have been estimated to be 6.8-16.3 cases per 100,000 persons and 14-42.7 cases per 100,000 persons.2-4 Its poor prognosis, combined with the scarcity of treatment options, provides a strong rationale for the development of novel therapeutic strategies. Many clinical trials with antifibrotic drugs for IPF are available in the last decade, including bosentan, imatinib, sildenafil, and interferon (IFN)-g-1b, but none of these have demonstrated a statistically significant treatment effect on the primary endpoint, except for several trials with pirfenidone5-10 and the INPULSIS trials with nintedanib.
The aim of this review was to estimate the efficacy and adverse events (AEs) of pirfenidone through a meta-analysis of published randomized controlled trials (RCTs).
Methods
Search strategy
MEDLINE, Cochrane Library, and ClinicalTrials.gov were searched for studies published before September 2015. This review was conducted according to the methods of Cochrane Collaboration review. All studies with the key words IPF or idiopathic pulmonary fibrosis or lung fibrosis and pirfenidone or Esbriet or PFD of published clinical trials of pirfenidone in IPF were collected.
Study selection. Inclusion and exclusion criteria
Inclusion and exclusion criteria were discussed to reach consensus within the reviewer team. Two investigators independently selected trials and extracted data in a nonblinded fashion. Differences in interpretations were resolved through discussions. Inclusion criteria were as follows: 1) data must be from an RCT study; 2) patients with IPF and aged 40-80 years, with diagnostic criteria conforming to the current guideline1-2; and 3) the dose of pirfenidone ≥1800 mg daily. Exclusion criteria were studies without outcome.
Methodological quality evaluation
Quality assessment and data extraction were conducted by 2 independent investigators. The methodological quality assessment was based on the Cochrane Reviewers’ Handbook11 and a modified Jadad scale.12 The original Jadad scale was a 5-point system, as the inadequate concealment of treatment allocation was associated with an exaggeration of treatment effects, it was decided to adopt a modified Jadad score scale, which assigned a maximum of 2 points for concealment.13 Under this system, a maximum score of 7 could be assigned. Studies with a score of ≥4 were considered to be high-quality studies. The data of modified Jadad score scale in this randomization, concealment, and blinded study were as follows: appropriate, 2 points; did not describe the details of randomization, 1 point; inappropriate, 0 point.
Statistical analysis
Five studies were included in this review. Stata 13 (Stata Corp, College Station, TX, USA) was used for meta-analysis. The outcome measures of these studies focused on the lowest oxygen saturation in the 6-min exercise test; the change from baseline to week 52 in the percentage of predicted forced vital capacity (FVC), vital capacity, and diffusing capacity for carbon monoxide (DLCO)%; progression-free survival (PFS), AEs (nausea, rash, photosensitivity reaction), and mortality (from any cause, related to IPF). The meta-analysis of the RCTs, hazard ratios (HR), relative risk (RR), and 95% confidence intervals (95% CIs) for pirfenidone versus placebo were performed. Twelve estimates and p-values were calculated using the DerSimonian-Laird method with the level of a=0.05. All statistical modeling procedures were completed using the statistical software R (3.1.1 for Win7 64 bit, Statistics Department of the University of Auckland, 1997) and Meta package (CRAN). Heterogeneity was estimated by performing a Cochrane-Q test and I-squared measure, which represented the variation in the RR that was attributable to heterogeneity. Significant heterogeneity was defined as a chi-squared test revealing p<0.114 or an I-squared value measuring > 50%.15 When the heterogeneity was not significant, a fixed-effects model was used to pool the results. The HR/RR and the 95% CI were pooled using the Mantel–Haenszel method. A random-effects model was used when the heterogeneity was significant. Publication bias was evaluated by performing the Begg’s funnel plot.
Study identification and selection
A total of 136 potentially relevant titles, abstracts, and articles were found. Initial screening resulted in 16 candidate studies.5-10,14 Detailed characteristics of the included RCTs are provided in Table 1. The five RCTs5,8-10,14 enrolled a total of 1568 participants (804 in the pirfenidone group and 764 in the placebo group) and were published between 2005 and 2014 (Table 1). Figure 1 shows the details of selection process and reasons for exclusion. After further screening, 5 trials met all inclusion criteria and were included in the final review.
Characteristics of included randomized controlled trials.
Literature search and selection, RCT - randomized controlled trials, IPF - idiopathic pulmonary fibrosis
Change in lung function
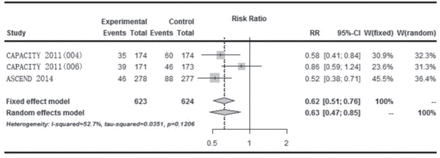
Change in FVC% ≥10% predicted In the SP3 study, a significantly smaller decline in FVC (0.09 L versus 0.16 L, p<0.0416) was seen in the high-dose pirfenidone treatment arm compared with placebo but the change in FVC% >10% predicted was not reported.6 The CAPACITY 1 and 2 phase III multinational randomized double-blind placebo trials were performed concurrently.8 The CAPACITY 1 study included 435 patients with high-dose pirfenidone (2403 mg per day), low-dose (1197 mg per day), and placebo. CAPACITY 2 study included 344 patients with only high-dose pirfenidone and placebo. A significant reduction in decline in FVC was found in the CAPACITY 1 study between high-dose pirfenidone and placebo arm (8% versus 12.4%, p=0.001). Primary endpoint was not met in the CAPACITY 2 study. Three trials (CAPACITY 1 and 2 and ASCEND) reported the change in FVC% ≥10% predicted. The forest plot showed that the change in FVC was statistically significantly different between the 2 groups favoring pirfenidone over placebo (RR: 0.62; 95% CI: 0.51-0.76, p<0.01) (Figure 2).
Forest plot showing comparison of the effect of pirfenidone versus placebo on change in forced vital capacity% ≥10%,
Change in DLCO
Azuma et al5 reported the change in DLCO. No significant difference was found between the 2 groups (p>0.05). In the CAPACITY trials, the difference between the 2 groups was also not statistically significant (p=0.301).
Lowest oxygen saturation in the 6-min steady-state exercise test
The lowest SpO2 during the 6-min steady-state exercise test was defined as clinical endpoint in 2 studies.5,6 The present meta-analysis showed no significant difference between pirfenidone and placebo in the lowest SpO2 during the 6-min steady-state exercise test [odds ratio (OR): 1.91; 95% CI: -1.71 to 5.00, p>0.05].
Progression-free survival
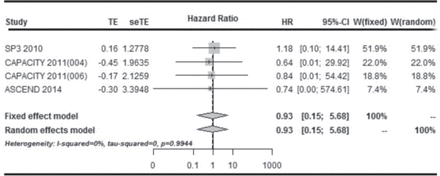
Three studies8,9 (CAPACITY 1; CAPACITY 2; ASCEND, 2014) reported the estimate of the hazard ratio (HR) as well as its 95% CI of PFS. Taniguchi6 did not report the values of HR and CI, which was instead indirectly estimated using data on the survival curves, such as percentage of PFS and number of patients at risk at different time points, and the P-value of the log-rank test.19 Therefore 4 studies6,8,⇓ (Taniguchi 2010; CAPACITY 1; CAPACITY 2; ASCEND, 2014) were included in the meta-analysis of PFS. The meta-analysis of the HR of PFS was performed using the fixed-effects model (P for heterogeneity = 0.994, I-squared = 0.00%) and revealed a significant reduction in the risk of progression in patients treated with placebo (HR: 0.93, 95% CI: 0.15–5.68; P = 0.99) (Figure 3).
Forest plot showing comparison of the effect of pirfenidone versus placebo on change in progression-free survival
Mortality
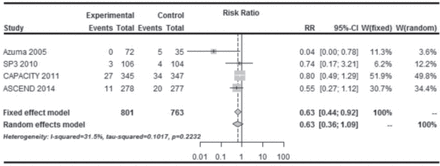
Four studies5,6,8,16 (CAPACITY 2011(1 and 2); ASCEND, 2014; Taniguchi 2010; Azuma 2005) reported the mortality from any cause. Pooled analysis showed a reduced mortality with pirfenidone, which was statistically not significant (OR: 0.63; 95% CI: 0.36–1.09; p=0.22) (Figure 4).
Forest plot showing comparison of the effect of pirfenidone versus placebo on mortality from any cause and mortality related to Idiopathic pulmonary fibrosis
Adverse events
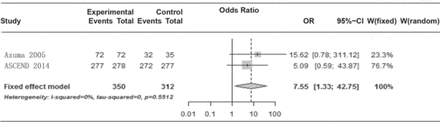
Almost all patients in the 5 studies5,6,8,16 (Azuma,2005; Taniguchi 2010; ASCEND, 2014;CAPACITY 1 and 2) reported at least one treatment-emergent AE. Two trials (Azuma, 2005; ASCEND, 2014) reported AEs. The present meta-analysis showed that the difference between the 2 groups was statistically significant (Figure 5). Two trials5,16 (Azuma, 2005; ASCEND, 2014) reported the incidence of nausea in the pirfenidone and the placebo arm, pooled analysis showed that the difference between the 2 groups was statistically significant (OR: 3.73; 95% CI: 2.48-5.62; p=0.75). Three trials (Azuma, 2005; SP3, 2010; CAPACITY 2011) reported that a significant number of patients receiving pirfenidone manifested a photosensitivity reaction. Pooled analysis showed that the difference between 2 groups was statistically significant (OR: 5.29; 95% CI: 1.45-19.30; p=0.004). Four trials (SP3, 2010; CAPACITY 1 and 2 2011; ASCEND, 2014) reported the incidence of rash. Pooled analysis showed that the difference between the 2 groups was statistically significant (OR: 2.95; 95% CI: 2.28-3.83; p=0.86).
Forest plot showing comparison of the effect of pirfenidone versus placebo on the change in the incidence of adverse events.
Discussion
This review assessed the efficacy and primary AEs of pirfenidone in treating IPF. Pooled analysis showed that pirfenidone reduced disease progression as assessed by the decline in FVC and improved PFS. Moreover, improved all-cause mortality was observed with pirfenidone treatment. forced vital capacity has been shown to be a reliable, valid, and responsive measurement of functional impairment in IPF, and the magnitude of change in FVC over time is highly predictive of survival.18-23 The slower decline in vital capacity and improvement in PFS may indicate a protective role of pirfenidone against disease progression in IPF.5-6,24-26 Reduction in disease progression may translate into a survival benefit for patients with IPF. In addition, the combined results of the trials revealed that the pirfenidone group had a significantly higher rate of AEs (nausea, rash, photosensitivity reaction) compared with the placebo group. A study showed that food intake might reduce the risk of certain AEs. Protective sun creams and avoiding exposure to direct sunlight may reduce photosensitivity reactions.27
The mortality rate in clinical trials with IPF is generally low. King et al4 analyzed 73 deaths occurring in the placebo arms during the follow-up of pirfenidone or interferon-gamma 1b trials for IPF. The all-cause mortality rate was low: only 6.6% at 1 year and 13.7% at 2 years. Due to this low event rate, every IPF trial in the past was underpowered to detect a statistically significant effect on mortality. In this study, pooled analysis showed that the difference between 2 groups was statistically significant in terms of mortality from any cause and mortality related to IPF. This indicated that pirfenidone could reduce mortality from any cause and mortality related to IPF.
Study limitations
First, the results might have been affected by publication bias as the number of included studies was still small. Second, heterogeneities among studies could confuse meta-analysis outcomes and might originate from different basic values. Finally, other frequently reported AEs, such as dyspepsia, dizziness, vomiting, anorexia, arthralgia, insomnia, abdominal distension, decreased appetite, stomach discomfort, weight reduction, abdominal pain, and asthenia, were not described and analyzed in detail in this study.8 Only 5 RCTs were included in this meta-analysis. Hence, large-sample studies are needed to validate the findings.
In conclusion, pirfenidone significantly reduced the progression of IPF, as measured by changes in FVC and PFS. The pirfenidone group was associated with a significantly higher rate of AEs (nausea, rash, photosensitivity reaction) compared with placebo, but the treatment was generally safe and the side-effect profile was acceptable. Hence, pirfenidone represents a suitable treatment option for patients with IPF.
Footnotes
Disclosure. Authors have no conflict of interest, and the work was not supported or funded by any drug company.
- Received March 13, 2017.
- Accepted July 25, 2017.
- Copyright: © Saudi Medical Journal
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.