Abstract
Objectives: To investigate the role of reactive-oxygen-species (ROS) induced epitopes on human-serum-albumin (HSA) and thyroid antigens in psoriasis autoimmunity.
Methods: This study was performed in the College of Medicine, Qassim University, Buraidah, Saudi Arabia between May 2014 and February 2015. The study was designed to explore the role of ROS-induced epitopes in psoriasis autoimmunity. Singlet-oxygen (or ROS)-induced epitopes on protein (ROS-epitopes-albumin) was characterized by in-vitro and in-vivo. Thyroid antigens were prepared from rabbit thyroid, and thyroglobulin was isolated from thyroid extract. Immunocross-reactions of protein-A purified anti-ROS-epitopes-HSA-immunoglobulin G (IgGs) with thyroid antigen, thyroglobulin, and their oxidized forms were determined. Binding characteristics of autoantibodies in chronic plaque psoriasis patients (n=26) against ROS-epitopes-HSA and also with native and oxidized thyroid antigens were screened, and the results were compared with age-matched controls (n=22).
Results: The anti-ROS-epitopes-HSA-IgGs showed cross-reactions with thyroid antigen, thyroglobulin and with their oxidized forms. High degree of specific binding by psoriasis IgGs to ROS-epitopes-HSA, ROS-thyroid antigen and ROS-thyroglobulin was observed. Immunoglobulin G from normal-human-controls showed negligible binding with all tested antigens. Moreover, sera from psoriasis patients had higher levels of carbonyl contents compared with control sera.
Conclusion: Structural alterations in albumin, thyroid antigens by ROS, generate unique neo-epitopes that might be one of the factors for the induction of autoantibodies in psoriasis.
Psoriasis, a chronic skin disorder is known to be the most prevalent autoimmune disorder in humans.1 It is characterized by hyperplasia of the epidermis, infiltration of leukocytes of dermis and epidermis as well as dilatation and proliferation of blood vessels, which are likely to be triggered by multiple factors such as drugs, physical and psychological stress, bacterial infections, or injury.2 Psoriasis appears in different clinical variants and the most frequently is the plaque psoriasis (also known as psoriasis vulgaris), presents with scaly red plaques on common areas, such as on scalp, the back, dorsal skin of the elbows, and ventral skin of knees.3 Although, the role of immunologic and environmental factors in the pathogenesis of plaques psoriasis has been proposed, but the precise etiology of disease remains poorly understood.1,3 It is well documented that oxidative stress is one of the major factors involved in the pathogenesis of psoriasis4-6 and now it has been well established that excess generation of reactive oxygen species (ROS) by the immune system play a vital role in the development of psoriasis.7 Cellular events such as cell proliferation, apoptosis, cell differentiation, and immune response are influenced by ROS, and these events are altered in psoriasis patients.4-7 Although the exact pathogenesis of psoriasis is unknown, but the occurrence of autoimmune reactions has been assumed,8-10 the presence of autoantibodies and various underlying immunologic abnormalities in the affected sites of these patients have also been reported.8,11-15 The autoimmune etiology has been also proposed on the basis of its association with various autoimmune diseases,8,10 but the precise mechanism of generation of autoantibodies in psoriasis remains unclear.
Thyroid disorders have a high prevalence in medical practice; they are associated with a wide range of diseases with which they may or may not share etiological factors. One of the organs which best show this wide range of clinical signs of thyroid dysfunctions is the skin.16-18 Thyroid abnormalities are well documented in psoriasis patients, thyroid gland causes an increase of epidermal growth factor levels, which has an important role in keratinocytes proliferation in psoriasis.19-21 In addition, a high prevalence of thyroid associated autoimmunity has also been reported in patients with psoriasis.20 Moreover, elevated ROS levels are often seen to be associated with thyroid dysfunctions, and now it is proposed that the thyroid hormones influence the ROS steady-state environment in the cell.22-24 The most common idea is the hyperthyroidism, which enhances the ROS production that perturbs the ROS steady-state environment to facilitate the cellular damage or damage to the cellular components as also reported in psoriasis patients.22,25 Therefore, it is assumed that in psoriasis, cells or cellular components are continuously exposed to oxidative stress, so that alterations in conformation and function of these cellular components may occur, which may results in modification of their biological properties. In view of these, this study was aimed to investigate the role of ROS-induced epitopes on albumin and thyroid antigens in psoriasis autoimmunity. To test this, ROS-modified epitopes were generated on albumin and antibodies against ROS-modified-albumin (anti-ROS-modified-epitopes antibodies) were experimentally generated. Cross-reactions of affinity purified anti-ROS-modified-epitopes immunoglobulin Gs (IgGs) with native- and ROS- modified thyroid antigen, thyroglobulin or human DNA were determined. Our data showed that anti-ROS-modified-epitopes-IgGs showed immunospecificity with thyroid antigen, thyroglobulin and with their oxidized forms. Importantly, the antigen(s) binding characteristics of naturally occurring chronic plaque psoriasis antibodies to ROS-modified epitopes, thyroid antigen, ROS-modified thyroid antigen, thyroglobulin, ROS-modified thyroglobulin, human DNA, and ROS-modified human DNA were determined.
Methods
Study design, patient’s recruitment, and literature search method
The study was performed in the College of Medicine, Qassim University, Buraidah, Saudi Arabia between May 2014 and February 2015. The present study was designed to investigate the role of ROS induced epitopes on HSA and thyroid antigens particularly thyroglobulin in psoriasis autoimmunity. The study was carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki as revised in Tokyo 2004) for humans and EC Directive 86/609/EEC for animals and was approved by Local Ethics Committee of College of Medicine, Qassim University, KSA. Study subjects were recruited through the dermatology outpatient clinics of Qassim University. Psoriasis was diagnosed after careful clinical examination of the patients and was classified as chronic plaque type in all studied groups. Inclusion criteria of the patients were based on the clinical diagnosis of psoriasis as defined by Johnson and Armstrong.26 Exclusion criteria of the patients were based on the following points: patients have active clinical evidence for infection during one week prior to the study, patients with chronic viral diseases, patients with cancer, patients with presence of hematologic, hepatic or renal disorders, pregnant patients. Venous blood samples from patients and normal human subjects were collected, and desired components from blood or serum were isolated on the same day of blood withdrawal for a patient or a control. The demographic details of patients and controls are summarize in Table 1.
Demographic and clinical details of 48 study subjects.
Preparation of protein-singlet oxygen antigen
Reactive oxygen species modified protein (protein-ROS) antigen was prepared in phosphate buffered saline (PBS) (10 mM sodium phosphate, 150 mM NaCl, pH 7.4) by generation of singlet oxygen as previously described27,28 with some modifications. Briefly, albumin (Sigma-Aldrich, Co, USA) (3.57 µM) in PBS was irradiated by 254 nm UV light for 30 min at room temperature in the presence of methylene blue (50 µM). The samples were dialyzed extensively with PBS, pH 7.4, to remove the dye. The singlet oxygen-induced epitopes on albumin was characterized by fluorescence spectroscopy and carbonylation.29-31
Induction of anti-ROS-modified-epitopes antibodies
The immunization of random bred, New Zealand white rabbits (2-2.5kg) was performed as described previously.31
Purification of immunoglobulin G
Immunoglobulin Gs (IgGs) from serum samples were isolated by affinity chromatography using Protein A-Agarose column (cat. # PA1-EA, Sigma-Aldrich) as described previously.31,32 The IgG concentration was determined considering 1.38 OD278=1.0 mg IgG/ml. The isolated IgG was dialyzed against PBS, pH 7.4, and stored at -80°C.
Preparation of thyroid and thyroglobulin antigens
Thyroid antigen was prepared from normal thyroid tissue obtained from rabbits. Immediately after removal, the thyroid tissue was washed with PBS, pH 7.4. After washing, the tissue was suspended in radio immunoprecipitation assay (RIPA) lysis buffer (catalog #20-188, Millipore, Temecula, CA, USA) and was homogenized. Homogenate was then centrifuged at 2000 g for 20 min, the pellet was discarded and the supernatant was dialyzed against PBS for 12 hours at 4ºC. Dialyzed material was used as thyroid antigen, whereas thyroglobulin antigen was prepared by gel exclusion chromatography of thyroid antigen using Sephacryl S-200 HR column (Pharmacia Fine Chemical, Uppsala, Sweden).
Deoxyribonucleic acid extraction
The DNA was extracted from whole blood of normal human subjects by MagNA Pure LC DNA Isolation Kit (cat. # 03003990001, Roche Applied Science, Mannheim, Germany) using MagNA Pure LC Automated Instrument according to the manufacturer’s instruction (Roche Applied Science, Mannheim, Germany). The absorbance of DNA solution was monitored at 260 nm and 280 nm to ascertain its purity and concentration.
Preparation of ROS-DNA, ROS-thyroid, and ROS-thyroglobulin antigens
Reactive-oxygen-species-modified antigens were prepared by UV irradiation (254 nm) of DNA, thyroid extract or thyroglobulin in the presence of methylene blue (50 µM) for 30 min at room temperature. The samples were dialyzed extensively in PBS, pH 7.4.27,28 The ROS-modified DNA was characterized by UV spectroscopy, whereas ROS-modified thyroid antigen or thyroglobulin was characterized by protein carbonyl contents estimation.30-32
Assay for protein oxidation
ROS induced protein oxidation in protein samples or patients’ sera was determined by protein carbonyl groups formation.30,31 Carbonyl contents were calculated by absorbance difference between test and control using the molar absorption coefficient of 22,000 M-1cm-1 at 370 nm. Protein concentration was determined in the samples and carbonyl contents were expressed as nmol/mg protein.
Enzyme-linked immunosorbent assays
Direct binding enzyme-linked immunosorbent assay (ELISA) was performed on flat bottom 96-well Nunc-ImmunoTM MicroWell polystyrene immunoplates (catalog # P8616, Sigma-Aldrich, St. Louis, MO., USA).29-33
Competitive binding assays
Antibody specificity was determined by competitive inhibition ELISA.34,35 Inhibitors were allowed to interact with a constant amount of affinity purified IgG samples for 2 hours at 37°C and overnight at 4°C. Formed immune complexes were coated in the wells instead of IgG samples. The remaining steps were the same in the direct binding ELISA.
Statistical analysis
Statistical comparisons were performed by One-way Analysis of Variance (ANOVA) followed by Tukey’s post-hoc analysis or 2-way ANOVA followed by Bonferroni post-hoc tests using Graph Pad Prism-5 (San Diego, CA, USA). P<0.05 was considered significant. Values shown are mean ± SEM unless stated otherwise.
Results
Characterization of singlet oxygen-modified epitopes
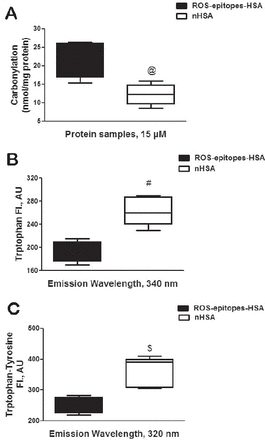
Albumin from human serum (HSA or albumin) was modified by singlet oxygen (or ROS), generated by the exposure of UV light on methylene blue. The singlet oxygen induced epitopes on albumin was studied by carbonyl contents formation. Our results showed that singlet oxygen-modified albumin adduct (ROS-epitopes-HSA) showed 53.3% increase in carbonyls contents as compared with its native analogue (p=0.0001; Figure 1A). Singlet oxygen-induced epitopes on albumin was also studied by tryptophan intrinsic fluorescence using an excitation wavelength 295 nm. Our results showed that fluorescence intensity (FI) at 340 nm of emission wavelength was 26.5% decreased, when the protein samples were excited at 295 nm (p=0.019; Figure 1B). The generation of epitopes on albumin by singlet oxygen was further re-confirmed by tryptophan-tyrosine fluorescence studies. Singlet oxygen-modified albumin showed 30.4% loss of FI at 320 nm of emission wavelength, when the protein was excited at 280 nm (p=0.021; Figure 1C).
Characterization of reactive oxygen species (ROS) induced epitopes. A) Carbonyl contents in ROS-modified human-serum-albumin (HSA) (ROS-epitopes-HSA) and unmodified HSA (nHSA). @p=0.0001 versus carbonyl contents present in nHSA. B) ROS induced tryptophan fluorescence alterations in ROS-epitopes-HSA. Fluorescence emission studies of ROS-epitopes-HSA and nHSA. The excitation wavelength was 295 nm. #p=0.0198 versus fluorescence intensity of ROS-epitopes-HSA. C) ROS induced tryptophan-tyrosine fluorescence alterations in ROS-epitopes-HSA. Fluorescence emission studies of ROS-epitopes-HSA and nHSA. The excitation wavelength was 280 nm. $p=0.021 versus fluorescence intensity of ROS-epitopes-HSA. Each whiskers box (min to max) represents the variance in 5 independent assays. The protein was in PBS, pH 7.4, and the concentration of all protein samples was 1 mg/ml. Comparison analysis was performed using the 2-way Analysis of Variance followed by Bonferroni’s post hoc test.
Antigenicity of singlet oxygen-modified epitopes
Antigenicity of singlet oxygen-induced epitopes was determined by immunizations of ROS-epitopes-HSA adduct or native albumin in rabbits. Reactive-oxygen-species-epitopes-HSA was found to be a strong antigen in rabbits inducing high titre antibodies (≥1:12800). Preimmune sera from the same rabbits serve as negative control, showed no binding with ROS-epitopes-HSA (data not shown). Immunization of rabbits with unmodified albumin under same experimental conditions showed low titre antibodies (1:3200; data not shown). Immunoglobulin G (IgG) against ROS-epitopes-albumin (anti-ROS-epitopes-HSA-IgG) was purified by affinity chromatography using Protein A-agarose affinity column. The purified IgG from immune and preimmune sera was found to elute in a singlet symmetrical peak and migrated as a singlet homogenous band on SDS-PAGE (data not shown). Direct binding ELISA of Protein A-agarose purified IgG from immune sera (or Immune IgG) showed strong binding with ROS-epitopes-protein, whereas IgG from preimmune sera (or preimmune IgG) showed negligible binding with ROS-epitopes-HSA under same experimental conditions (data not shown).
Detection of singlet oxygen-modified epitopes by chronic plague psoriasis patients’ IgGs
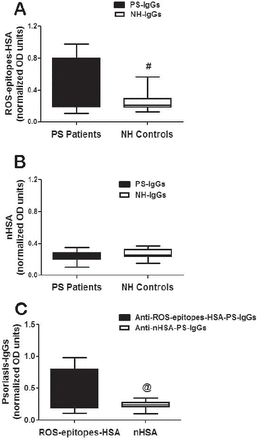
In an attempt to understand the role of singlet oxygen (ROS) generated epitopes in the pathogenesis of psoriasis (PS), this is the first report, which determined the role of ROS-modified epitopes protein and its associated autoimmunity in the pathogenesis of PS, 26 AA patients’ sera were selected and immunoglobulin Gs (IgGs) were purified from all selected patients by affinity chromatography. The purified IgG was eluted in a symmetrical peak (data not shown). Direct binding ELISA with these affinity purified IgGs showed 46.1% of IgG samples from PS patients (PS-IgG) showed strong binding to ROS-epitopes-HSA over IgG from normal human subjects (NH-IgG) (Figure 2A). The average normalized absorbance at 450 nm (±SEM) of the 26 PS-IgG binding to ROS-epitopes-HSA was 0.50±0.06 and the 22 NH-IgG was 0.24±0.02. A p<0.05 (p=0.021) indicates that IgG from patients showed strong binding with ROS-epitopes-HSA over NH-IgGs. Specificity of affinity purified IgGs from PS patients or NH subjects were also screened towards unmodified albumin (nHSA). Both PS-IgG and NH-IgG showed negligible binding to nHSA (p=0.26). The average normalized absorbance (±SEM) at 450 nm of the 26 PS-IgG binding to nHSA was 0.24±0.01 and the 22 NH-IgG was 0.26±0.01, (Figure 2B). Moreover, we also determined the difference in the recognition of ROS-epitopes-HSA and nHSA by psoriasis autoantibodies (Figure 2C). Our data reveal marked difference in the binding of ROS-epitopes-HSA and nHSA by PS-IgGs (p=0.0005).
Direct binding of psoriasis immunoglobulin G to reactive oxygen species (ROS)-epitopes-human-serum-albumin (HSA). A) Levels of anti-ROS-epitopes-HSA-IgGs in psoriasis (PS) patients (n=26) and normal human (NH) controls (n=22). Anti-ROS-epitopes-HSA-PS-immunoglobulin G (IgG) from PS patients’ sera specific to ROS modified HSA (ROS-epitopes-HSA); anti-ROS-epitopes-HSA-NH-IgGs stands for IgG from NH controls’ sera specific to ROS-epitopes-HSA. Black whiskers box (min to max) represents the variance in 26 independent assays, whereas whiskers box (min to max) represents the variance in 22 independent assays. #p=0.021 versus anti-ROS-epitopes-HSA-PS-IgGs. B) Levels of anti-nHSA-IgGs in PS patients (n=26) and NH controls (n=22). Anti-nHSA-PS-IgGs stands for immunoglobulin G from PS patients’ sera specific to native HSA (nHSA); anti-nHSA-NH-IgGs stands for IgG from NH controls’ sera specific to nHSA. Black whiskers box (min to max) represents the variance in 26 independent assays, whereas whiskers box (min to max) represents the variance in 22 independent assays. Anti-nHSA-PS-IgGs versus anti-nHSA-NH-IgGs, p=0.261. C) Levels of anti-ROS-epitopes-HSA-IgGs and anti-nHSA-IgGs in PS patients (n=26). Both whiskers boxes (min to max) represent the variance in 26 independent assays. Microtitre plates were individually coated with ROS-epitopes-HSA or nHSA (10 µg/ml). @p=0.0005 versus ROS-epitopes-HSA. Comparison analysis was performed using the 2-tail test followed by Mann Whitney post hoc analysis.
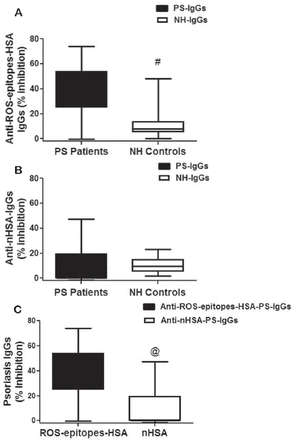
Binding of psoriasis antibodies to ROS-epitopes-HSA was further investigated by competitive binding assays in 26 PS patients. The interaction of ROS-epitopes-HSA with PS-IgG was ascertained by direct binding assays. Figure 3A shows competitive assays of different PS-IgG and NH-IgG by ROS-epitopes-HSA. Most of the tested PS-IgG showed strong recognition of ROS-epitopes-HSA as compared with the tested NH-IgG (p=0.021). The average percent inhibition (±SEM) of the 26 PS-IgGs towards ROS-epitopes-HSA was 38.5±2.6 and the 22 NH-IgGs was 10.9±1.9 (Figure 3A). In addition, experiments were also performed for the recognition of nHSA by PS-IgGs or NH-IgGs. PS-IgGs or NH-IgGs under same experimental conditions showed insignificant recognition of nHSA (p=0.079). The average percent inhibition (±SEM) of the PS-IgG towards nHSA was 11.0±2.1 and NH-IgG was 10.3±0.9 (Figure 3B). Furthermore, we also demonstrated the difference in the recognition of PS-IgG to ROS-epitopes-HSA and nHSA (Figure 3C). Immunoglobulin G from patients showed strong recognition of ROS-epitopes-HSA as compared with unmodified albumin (p=0.0005).
Competitive binding assays of psoriasis immunoglobulin G (IgGs) to reactive-oxygen-species (ROS)-epitopes-human-serum-albumin (HSA). A) Inhibition of IgGs from psoriasis patients (PS) and normal human (NH) controls by ROS modified HSA (ROS-epitopes-HSA). #p=0.021 versus PS patients. Black whiskers box (min to max) represents the variance in 26 independent competitive binding assays, whereas whiskers box (min to max) represents the variance in 22 independent competitive binding assays. B) Inhibition of IgGs from PS and NH controls by native HSA (nHSA). Psoriasis patients versus NH controls, p=0.079. C) Inhibition of psoriasis IgGs by ROS-epitopes-HSA and nHSA. Both whiskers boxes (min to max) represent the variance in 26 independent competitive binding assays. Microtitre plates were individually coated with ROS-epitopes-HSA or nHSA (10 µg/ml). @p=0.0005 versus ROS-epitopes-HSA. Comparison analysis was performed using 2-tailed test followed by Mann Whitney post hoc analysis.
Human DNA, thyroid antigen, thyroglobulin and their singlet oxygen-modified conformers
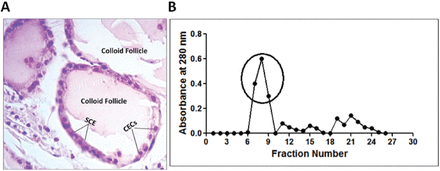
Deoxyribonucleic acid was isolated from whole blood of normal healthy individuals. The purity (A260/A280 ratio = 1.8-1.9) and concentration of the DNA preparations were ascertained by A260 and A280 measurements. Whereas, thyroid antigen was prepared from thyroid tissue obtained from rabbits. Examination of the thin section obtained from the rabbit thyroid glands showed the normal histological structure. The thyroid gland was formed of colloid follicles of various sizes, each follicle was lined with a singlet layer of simple cuboidal epithelium (SCE), filled with cuboidal epithelial cells (CECs) (Figure 4A). Thyroglobulin was prepared from thyroid extract by gel exclusion chromatography using Sephacryl S200 HR column. Thyroglobulin was eluted in the initial peak of the elution profile of thyroid extract, therefore the eluted protein in the initial peak was used as thyroglobulin (Figure 4B).
A) Histopathology of thyroid tissue. Hematoxylin and eosin staining of thyroid tissue dissected from rabbit (400X). B) Preparation of thyroglobulin. Gel exclusion chromatography of rabbit thyroid extract on Sephacryl S200 HR column. Indicated fractions in circle were collected and were used as thyroglobulin. SCE - simple cuboidal epithelium, CECs - cuboidal epithelium cells.
Singlet oxygen induced modification in human DNA was studied by UV absorbance spectroscopy. Singlet oxygen (or ROS)-modified human DNA showed 38.8% hyperchromicity at 260 nm under same experimental conditions of unmodified human DNA. Modifications in thyroid antigen and thyroglobulin by singlet oxygen have been studied by carbonyl contents estimation. The average carbonyl contents (±SD) of 5 independent assays in unmodified thyroid antigen were 19.9±4.2 nmol/mg protein. Whereas, the carbonyl contents (±SD) of 5 independent assays in singlet oxygen-modified thyroid antigen were 32.8±6.9 nmol/mg protein, a p<0.001 (p=0.0009) indicates significant difference in the carbonyl contents of unmodified and modified thyroid antigens. Similarly the carbonyl contents were also estimated in native and ROS modified thyroglobulin. The average carbonyl contents (±SD) of 5 independent assays in native thyroglobulin was 12.8±2.1 nmol/mg and singlet oxygen-modified thyroglobulin was 25.5±3.9 nmol/mg protein. A p<0.01 (p=0.0073) indicates that carbonyl contents were significantly increased in modified thyroglobulin. The percentage modification of thyroid antigen, thyroglobulin, and human DNA is summarize in Table 2.
Characterization of singlet oxygen induced modifications inthyroid antigen, thyroglobulin, and human DNA.
Recognition of thyroid antigen, ROS-modified thyroid antigen, thyroglobulin and ROS-modified thyroglobulin by anti-ROS-epitopes-protein-IgGs
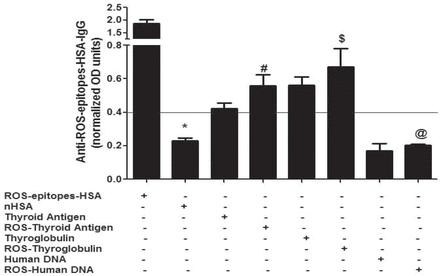
Affinity purified anti-ROS-epitopes-HSA-IgGs were tested for binding to thyroid antigens, human DNA, and their singlet oxygen (or ROS)-modified forms. Our direct binding assays showed that thyroid antigen, thyroglobulin, and their oxidized forms were well recognized by anti-ROS-epitopes-HSA-IgGs, but the recognition was more with ROS-thyroid antigen or ROS-thyroglobulin. Whereas, human DNA and its oxidized form showed no binding with anti-ROS-epitopes-HSA-IgGs. Reactive-oxygen-species-epitopes-HSA showed strong binding with anti-ROS-epitopes-HSA-IgGs and was used as a positive control, whereas native HSA showed negligible binding with anti-ROS-epitopes-HSA-IgGs and was used as negative control (Figure 5).
Recognition of thyroid antigens and their oxidized forms by anti-reactive-oxygen-species (ROS)-epitopes-human-serum-albumin (HSA)-immunoglobulin G (IgGs). Binding characteristics of affinity purified anti-ROS-epitopes-HSA-IgGs to thyroid extract, ROS-modified thyroid antigen (ROS-thyroid antigen), thyroglobulin, ROS-modified thyroglobulin (ROS-thyroglobulin), human DNA, ROS-modified human DNA (ROS-human DNA) determined direct binding ELISAs. Reactive-oxygen-species modified HSA (ROS-epitopes-HSA) was used as a positive control, and native HSA (nHSA) was used a negative control for anti-ROS-epitopes-HSA-IgGs. Values above 0.4 (y axis) were considered positive. Each histogram represents as a mean ± SEM. *p=0.000 versus ROS-epitopes-HSA; #p=0.013 versus thyroid antigen; $p=0.008 versus thyroglobulin; @p=0.218 versus human DNA.
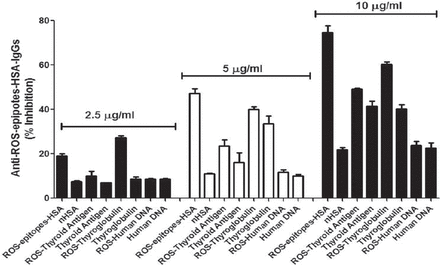
The antigenic specificity of induced anti-ROS-epitopes-HSA-IgGs was further characterized by competitive assays (Figure 6). A maximum of 74.7% inhibition of the anti-ROS-epitopes-HSA-IgGs with the immunogen as inhibitor was observed at 10 µg/ml of inhibitor concentration. The anti-ROS-epitopes-HSA-IgGs exhibited a variable recognition of thyroid antigen, ROS-thyroid antigen, thyroglobulin and ROS-thyroglobulin in competition ELISA. Inhibition of anti-ROS-epitopes-HSA-IgGs by thyroid antigen was 41.4%, ROS-thyroid antigen 49.9%, thyroglobulin 40.2%, and ROS-thyroglobulin 60.2% at 10 µg/ml of inhibitor concentration. Whereas, anti-ROS-epitopes-HSA-IgGs were not recognized human DNA, ROS-human DNA, and native albumin. The pattern of anti-ROS-epitopes-HSA-IgGs recognition was same at 2.5 or 5 µg/ml of inhibition concentration (Figure 6).
Competitive inhibition of anti-reactive-oxygen-species (ROS)-epitopes-human-serum-albumin (HSA)-immunoglobulin G (IgGs). Inhibition of affinity purified anti-ROS-epitopes-HSA-IgGs by thyroid antigen, ROS-modified thyroid antigen (ROS-thyroid antigen), thyroglobulin, ROS-modified thyroglobulin (ROS-thyroglobulin), human DNA, ROS-modified human DNA (ROS-human DNA) determined by competitive binding assays. Microtitre plates were coated with ROS-epitopes-HSA (10 µg/ml), whereas thyroid extract, ROS-thyroid extract, thyroglobulin, ROS-thyroglobulin, human DNA, ROS-human DNA were used as inhibitors with different concentrations (2.5-10 µg/ml). Each histogram represents a mean ± SEM of 5 independent assays. nHSA - native human-serum-albumin
Recognition of thyroid antigen, ROS-thyroid antigen, thyroglobulin, ROS-thyroglobulin by psoriasis antibodies
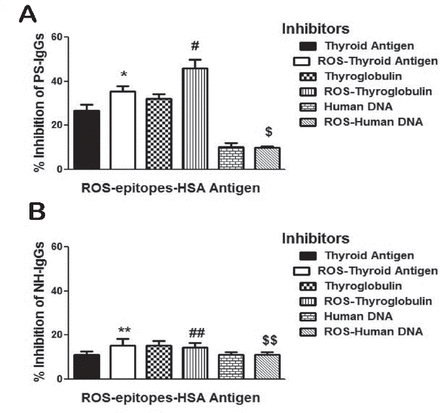
The specificity of protein-A purified psoriasis IgGs (PS-IgGs) from selected sera of psoriasis patients was evaluated by competitive inhibition ELISA using thyroid antigen, ROS-thyroid antigen, thyroglobulin, ROS-thyroglobulin, human DNA, and ROS-human DNA as inhibitors (Figure 7A). Enzyme-linked immunosorbent assay plates were coated with ROS-epitopes-HSA used as antigen in the competitive binding assays. Results showed higher reactivity of PS-IgGs towards ROS-thyroglobulin, ROS-thyroid antigen, thyroglobulin and thyroid antigen, whereas native and ROS-modified human DNA didn’t recognized by PS-IgGs. The average percentage inhibition (±SEM) in the binding of 5 PS-IgGs to ROS-epitopes-HSA by thyroid antigen was 26.8±2.7, ROS-thyroid antigen 35.4±2.5, thyroglobulin 32.2±2.0, ROS-thyroglobulin 46.0±3.9, human DNA 10.0±1.88, and ROS-human DNA 9.8±0.7. Moreover, we have also tested affinity purified normal human antibodies for the recognition of the same thyroid antigen, thyroglobulin, human DNA, and their oxidative forms under same experimental conditions as with PS antibodies (Figure 7B). Results revealed that NH IgGs showed negligible binding to all the tested inhibitors (p>0.05). Data pointed out an enhanced recognition of thyroglobulin and thyroid antigen and their oxidized forms by PS antibodies, when compared with the antibodies from healthy controls (Figure 7).
Competitive inhibition of psoriasis IgG. Inhibition of affinity purified psoriasis immunoglobulin G (PS-IgG) A) and normal human IgG (normal human [NH]-IgG) B) binding to reactive-oxygen-species (ROS)-epitopes-HSA antigen. Inhibitors used were thyroid extract, ROS-modified thyroid extract (ROS-thyroid extract), thyroglobulin, ROS-modified thyroglobulin (ROS-thyroglobulin), human DNA and ROS-modified human DNA (ROS-human DNA). Inhibitors concentration used in the competitive binding assays was 20 µg/ml. Microtitre plates were coated with ROS-epitopes-HSA (10µg/ml). Each histogram represents a mean ± SEM of four independent assays. *p=0.004 versus thyroid antigen, #p=0.043 versus thyroglobulin, $p=0.845 versus human DNA, **p=0.265 versus thyroid antigen, ##p=0.778 versus thyroglobulin,$$p=1.000 versus human DNA.
Protein oxidation in psoriasis patients
Protein carbonyl contents have now considered being an excellent biomarker of protein oxidation; therefore, carbonyl contents were investigated in the serum samples of psoriasis patients and their results were compared with healthy human controls. The data showed significant increase in serum protein carbonyl contents in PS patients compared with healthy human controls (p=0.002). The average carbonyl contents (±SD) in PS patients’ sera (n=26) was 2.75±0.84 nmol/mg protein and controls’ sera (n=22) 1.86 ± 0.69 nmol/mg protein (data not shown).
Discussion
It is well documented that HSA serves as a biomarker of oxidative stress, as its redox alteration modulates its physiologic functions.29,31 In the present study, HSA was modified by singlet oxygen (or ROS) in-vitro. Reactive-oxygen-species induced oxidative modification was studied by protein carbonyl formation.29-31 Formation of protein carbonyl contents is the most commonly used reliable marker of protein oxidation.36 Singlet oxygen induced structural alterations in HSA was studied by tryptophan intrinsic fluorescence.29-31 Albumin from human contains only one tryptophan residue (Trp-214), which is present in domain-II. The modification in tryptophan residue was confirmed by the loss in fluorescence intensity when the protein samples were excited at 295 nm, this clearly indicating that singlet oxygen induced structural alterations was occurred in domain-II of albumin. These alterations in the structure were further validated by exciting the albumin samples at 280 nm. Singlet oxygen induced alterations in HSA was further re-validated in-vivo by immunization of ROS-epitopes-HSA and unmodified HSA in rabbits. Reactive-oxygen-species-epitopes-HSA was found to be a powerful antigenic stimulus inducing high titre antibodies in rabbits, whereas unmodified albumin induced low titre antibodies under same experimental conditions. The enhanced antigenicity of ROS-epitopes-HSA in comparison with unmodified albumin could possibly be due to the generation of potential neo-epitopes against, which antibodies were raised.
Psoriasis is an immune-mediated skin disorder and its pathogenesis is complex, where multiple exogenous and endogenous factors are involved.1-3,37,38 Despite the availability of modern molecular approaches but still pathogenesis of psoriasis remains to be fully investigated. However, now it is well established that oxidative stress plays an important role in psoriasis as well as in other skin disorders.4-6,39 Various studies have shown that psoriasis is associated with increased formation of ROS and decrease in antioxidant potential.4-6,39 Therefore, this may cause oxidative damage of cellular components including proteins and nucleic acids directly or indirectly, which could results in the modification of their biological properties or may lead to the formation of neoantigens, which could in turn initiate autoimmunity. In the present study, the binding characteristic of naturally occurring psoriasis autoantibodies to ROS-epitopes-HSA was investigated. Sera of 26 patients with chronic plaque psoriasis and 22 normal human subjects were selected and immunoglobulin Gs (IgGs) were isolated using Protein-A affinity chromatography. Of these, 42.3% patients IgGs showed preferentially high binding to ROS-epitopes-HSA as compared to its native analogue. No appreciable binding was observed with the normal human controls’ IgGs. To have a better insight into the recognition of psoriasis antibodies with native and ROS-epitopes-HSA, IgGs from patients were cross examined by competitive inhibition assays. Competition ELISA with IgGs from patients (PS-IgGs) reiterated the direct binding results, that the ROS-epitopes-HSA is an effective inhibitor for PS-IgGs, showing substantial difference in the recognition of ROS-epitopes-HSA over unmodified HSA. Whereas, IgGs from normal human controls neither showed binding with ROS-epitopes-HSA or nor with native HSA. Employing various immunological techniques, the data clearly demonstrate a substantial increase in the recognition of ROS-epitopes-HSA over unmodified HSA by circulating psoriasis autoantibodies.
In spite of the fact that recent advancements have been reported in the treatment and pathogenesis of psoriasis, but still its etiology remains obscure. Many endocrinological abnormalities are assumed to exacerbate psoriasis.19 An improvement in psoriasis is reported in patients with psoriatic hyperthyroidism.19 Moreover, it has been shown that 2 thyroid hormones (T3 and T4) cause an increase in epidermal growth factor (EGF), which leads to epidermal hyperplasia.16-19 Although the mechanism of action of these anti-thyroid preparations is unclear, but clearly indicated that thyroid constituents have their definite role in the pathogenesis of psoriasis.16-20,40 For these reasons, we have tested the specificity of experimentally induced antibodies against ROS-modified epitope(s) towards thyroid antigens and their oxidized forms. Not only these, we have also tested the specificity of induced antibodies towards native and ROS modified human DNA. Our results showed that human DNA and its oxidized form were not recognized by affinity purified anti-ROS-epitopes-HSA-IgGs. However, affinity purified anti-ROS-epitopes-HSA-IgGs showed cross reactions with thyroid antigen, ROS-modified thyroid antigen, thyroglobulin, and ROS-modified thyroglobulin. Therefore, it is thought worthwhile to investigate the binding characteristics of natural occurring psoriasis autoantibodies to thyroid antigens and their oxidized forms, so that the possible involvement of their role in psoriasis autoimmunity could be ascertained. Our novel data showed that affinity purified PS-IgGs showed strong recognition of thyroid antigen, thyroglobulin and their oxidized forms, when compared with the antibodies from normal human controls. More specifically, our data also pointed that IgGs from patients recognized ROS-modified thyroid antigens (ROS-thyroid antigen and ROS-thyroglobulin) in a greater extent as compared with unmodified thyroid antigens (thyroid antigen and thyroglobulin). The substantially enhanced immunogenicity of ROS-modified thyroid antigens in psoriasis patients could possibly be due to the ROS-generated neo-epitopes on thyroid antigen that might play a role in the induction of circulating autoantibodies in psoriasis. Moreover, oxidative damage of proteins in psoriasis patients was further re-confirmed by quantification of protein carbonyl contents in patients, and normal human studied subjects. As it is well reported that oxidation of proteins typically results in an increase in carbonyl contents, which can serve as in-vivo biomarkers of oxidative stress.36 In the present study, total serum protein carbonyl content was found to be significantly higher in psoriasis patients as compared with healthy human controls. These results strongly support our central hypothesis that proteins in psoriasis patients are oxidatively modified namely, the structural perturbation of proteins by excess generation of singlet oxygen (or ROS) rendering them immunogenic. The generated neo-epitopes might play a role in the induction of circulating autoantibodies in psoriasis.
Study limitations
The most obvious limitation of the study is the sample size and region of sample collection. It will be better to include 40-80 patients and also sample collection should not be confined to only one region. In addition, diverse antigen characteristic of in-vivo generated anti-ROS-epitopes-antibodies with more psoriasis associated antigens will further strengthen to our findings.
In conclusion, our novel data demonstrated that exposure of singlet oxygen (or ROS) caused oxidative damaged to proteins and presents unique epitopes for the generation of autoantibodies specific to thyroid antigens particularly thyroglobulin, and their oxidized forms. Blood proteins and thyroid antigens modified with singlet oxygen may be important factors for the induction of circulating psoriasis autoantibodies. These findings contribute to a paradigm of protein oxidation and oxidative-by- products causing the generation of autoantibodies in autoimmune diseases.
Withdrawal policy
By submission, the author grants the journal right of first publication. Therefore, the journal discourages unethical withdrawal of manuscript from the publication process after peer review. The corresponding author should send a formal request signed by all co-authors stating the reason for withdrawing the manuscript. Withdrawal of manuscript is only considered valid when the editor accepts, or approves the reason to withdraw the manuscript from publication. Subsequently, the author must receive a confirmation from the editorial office. Only at that stage, authors are free to submit the manuscript elsewhere. No response from the authors to all journal communication after review and acceptance is also considered unethical withdrawal. Withdrawn manuscripts noted to have already been submitted or published in another journal will be subjected to sanctions in accordance with the journal policy. The journal will take disciplinary measures for unacceptable withdrawal of manuscripts. An embargo of 5 years will be enforced for the author and their co-authors, and their institute will be notified of this action.
Acknowledgment
The authors would like to thank Mr. Casimero A. Victoria and Mr. Muhammad El-Safi for their help in some experimentation. Thanks are also due to our veterinarian Dr. Sayed E. Mahana for the maintenance of experimental animals during the study period.
Footnotes
Disclosure. Authors have no conflict of interests, and the work was not supported or funded by any drug company. This work was funded by Qassim University Research Deanship, Qassim, Saudi Arabia (Grant SR-D-014-2551).
- Received June 11, 2015.
- Accepted October 23, 2015.
- Copyright: © Saudi Medical Journal
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.