Abstract
Objectives: To develop and establish research guidelines of international standards that matches appropriate cross-cultural and religious considerations of the region.
Methods: We followed the guidelines given by the American College of Cardiology Foundation and American Heart Association, Inc., Washington DC, USA task force on practice to develop research guidelines of international standards for our institution. In October 2015 the task team (of a private higher education institution in Jeddah, Saudi Arabia) qualitatively analyzed the national codes and guidelines using 19 ethical protections stated in the other universally accepted international guidelines.
Results: The electronic search yielded 11 different guidelines and documents, of which 4 documents were from Saudi Arabia. When the Saudi Arabian documents were compared using 19 universally accepted ethical protections, we found one of the document contained 15 of the 19 ethical protections, another document contained 9 protections and the least being 5 of the 19 protections.
Conclusion: Research guidelines of international standards were developed with respect to the cultural, traditional, and religious considerations of the Kingdom, providing a valuable framework to guide our institutional researchers to conduct ethically sound inquiry.
The Kingdom of Saudi Arabia (KSA) has recently witnessed a surge in the establishment of new private higher education institutions. These institutions have adopted 3 traditional components in their mission statements namely; teaching, research, and community services.1 According to Darandari et al,2 between 2005 and 2008, the National Commission for Academic Accreditation and Assessment developed and established quality assurance and accreditation system to assist program and institutional self-evaluation in KSA. This and other such bodies require mandatory institutional research for accreditation of higher education institutions. Resources and research guidelines of international standards are the building blocks that underpin the development of institutional research. The research guidelines aim at safeguarding the rights, well-being, and dignity of the research participants, and imposes an obligation to abide-by the moral commitment to the advances in health care, science and technology.3 Vital to its mission commitment to research, Ibn Sina National College for Medical Studies, Jeddah, Saudi Arabia established a research center with the primary goal to facilitate research of its faculty members and students in the field of health sciences. The research center set-out to establish research guidelines for the research community of the institution with following 2 objectives: 1) develop research guidelines of international standards, and 2) the guidelines should match the appropriate cross-cultural and religious considerations of the region. This report details the process of developing and establishing research guidelines at a private higher education institution of Saudi Arabia. Furthermore, this report could be a valuable guide to other similar institutions of the region to develop and establish their research guidelines.
Methods
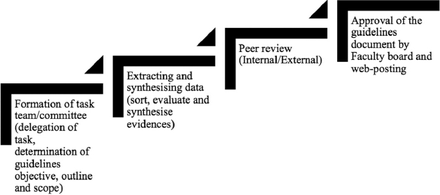
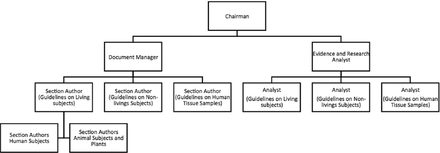
In October 2015, a task team or committee was formed from the members of the research center in Ibn Sina National College for Medical Studies, Jeddah, Saudi Arabia. The task of compiling the guidelines was a joint activity carried out by the team members representing the 4 health science streams of the institution. The task team followed the steps (Figure 1) recommended by the American College of Cardiology/American Heart Association task force on practice guidelines4 to facilitate the workflow in accomplishing the task. The work was approved by Ibn Sina National College for Medical Studies Institutional Ethics Committee. The organizational structure of the task force team is showed in Figure 2. Broadly, the task force comprised 2 major categories of members namely: 1) the document mangers, 2) and evidence analysts. The roles and contributions of the task team members are detailed in Table 1.
Guidelines development process chart.
Organizational structure of the Task Force Team, modified from Methodology Manual and Policies from the American College of Cardiology Foundation and American Heart Association, Inc. Task Force on practice guidelines.
Membership, roles, and responsibilities of task teams.
Data extraction and synthesis
While extracting and synthesizing data, we used the method developed by Alahmad et al:5 1) Electronically search for international and regional guidelines through the websites and web pages that provided direct access. 2) Select documents that included national and international codes and guidelines in English that solely dealt with research ethics. 3) Qualitatively describe the selected codes and guidelines for explicit description of research regulations and compare against the 19 ethical protections stated in the other universally accepted international documents such as the Declaration of Helsinki, International Conference of Harmonization - Guidelines for Good Clinical Practice (ICH-GCP),6 Council for International Organizations of Medical Sciences (CIOMS)7 guidelines and UNESCO Universal Declaration on Bioethics and Human Rights8 (Table 2). 4) Determine the purpose of the codes and guidelines documents selected and ascertain whether the research ethics protocols matched the regional settings.5
Ethical protections stated in research ethics documents of Saudi Arabia modified from BMC Medical Ethics. Alahmad G, Al-Jumah M, Dierickx K. Review of national research ethics regulations and guidelines in Middle Eastern Arab countries. BMC Med Ethics 2012; 13: 34.
The guidelines document was compiled (Figure 1); it was first internally peer-reviewed before requesting an external review. Any recommendations that mandated modifications to the guidelines as a result of the peer review were balloted for consensus before modification.
Finally, establishing guidelines requires endorsement by the authorities to reinforce the credibility and successful implementation. Therefore, the research guidelines were ratified by the faculty board; the highest governing council of the institution before web posting.
Results
The electronic search for international and regional guidelines yielded 11 different guidelines and documents: the Nuremberg code,9 the World Medical Association (WMA) Declaration of Geneva, the WMA Declaration of Lisbon on the Rights of the Patient, the WMA Declaration of Helsinki-Ethical Principles for medical research involving human subjects,10 the ICH-GCP of 1996,6 the CIOMS International Ethical Guidelines for Biomedical Research involving human subjects,7 the UNESCO Universal Declaration on Bioethics and Human Rights,8 the “Ethics of the Medical Profession” (1998; renewed, 2007),11 Saudi Food and Drug Administration (SFDA), Clinical Trial Requirement Guidelines (2009),12 the Law of Ethics of Research on Living Creatures (article 321) of the ministers’ council to the King of Saudi Arabia, and the Implementing Regulations of the Law of Ethics of Research of Living Things.13
Among the 11 documents, 3 were from WMA and 4 from Saudi Arabia. The 4 Saudi Arabian documents contained clear reference to the regulation of research ethics. The first document (I) is a general guide that contains a chapter on biomedical research for both humans and animals, with specific reference to the main principles of clinical research. The second document (II) referred to the clinical studies which are conducted in human subjects. The third document (III) is a law stated by the council of ministers, and the fourth document (IV) describes the implementation of the law. The Saudi Arabian document II contained 15 out of 19 ethical protections of international guideline provisions used for comparison, followed by document III that contained 9 protections, and lastly, document I contained 5 protections only.
Discussion
Health science research may involve living creatures (humans, animals, and plants). Therefore to protect the rights and welfare (the appropriate care and respect) of the research subjects, a comprehensive and organized program (research guidelines) is mandatory to be in practice.
Looking at the evolution of research ethics and regulations, efforts by international bodies such as World Health Organization, UNESCO and the like to regulate the medical experiments on human subjects prior to World War II were very limited. Post-world War II, several countries, and international organizations have developed a number of regulations and codes of research,5 in that, the Nuremberg Code of 1947 can be considered the most up-to-date document.9 Later, a number of international guidelines were laid down, such as, the Declaration of Geneva in 1948 (later amended in 1968, 1983, and 1994), the Declaration of Patient Rights produced by the WMA in Lisbon, the Declaration of Helsinki on ethical principles for medical ethics and human rights (adopted 1964; latest amendment 2013) was developed by the WMA,10 and the International Conference of Harmonization-guidelines for good clinical practice (ICH-GCP) in 1996.6 In 2002, the CIOMS proposed the International Ethical Guidelines for biomedical research involving human subjects,7 and 2005 observed the adoption of the UNESCO Universal Declaration on bioethics and human rights.8 Although, the above mentioned guidelines propose a general framework of basic values for medical ethics that are considered to be universally shared by many other similar bodies’ worldwide, they lack the factual and appropriate cross-cultural and religious variations.3,14 A literature search for research guidelines that match the local settings of the Middle East yielded very few results. A recent study of the region published by Alahmad et al,5 entitled “Review of national research ethics regulations and guidelines in Middle Eastern Arab Countries” concluded that although enormous efforts have been made, only a few countries in the region have developed their own research guidelines.5 However, in the Saudi Arabian context, the Law of Ethics of Research on Living Creatures (article 321, by the ministers’ council to the King of Saudi Arabia in 2010, enacted and approved by Royal Decree No. (M/59) on 14/09/1431 Hijri (24/08/2010) and the implementing regulations of the Law of Ethics of Research of Living Things (in 20/01/1433) Hijri (25/12/2011) aim at establishing a foundation and necessary regulations to conduct research on living creatures without contradicting Islamic concepts and beliefs.13 By this, KSA has successfully linked Law of Ethics of Research on Living Creatures and the implementing regulations with Sharia (the law adopted by official bodies in KSA). Alahmad et15 al used a framework based on 7 criteria to examine the Law of Ethics of Research on Living Creatures and their implementing regulations, and suggested these could be a useful reference while developing the research ethical guidelines in the region.15
While developing the guidelines, we considered 2 categories of guidelines and documents namely; 1) the international guidelines and documents, and 2) Saudi Arabian guidelines and documents. The international guidelines and documents include the provisions stated in the Declaration of Helsinki the ICH-GCP and the UNESCO Universal Declaration on Bioethics and Human Rights, owing to their universal acceptance worldwide. We also included the CIOMS guidelines, because these were drafted specifically to implement the Declaration of Helsinki in the developing countries and to address the implications in multinational or transnational research in which the developing countries may be partners.7 In addition, the Islamic Organization of Medical Sciences confirmed that CIOMS guidelines were fully compatible with the Islamic law.16 The Saudi Arabian guidelines and documents include, the law and the Implementing Regulations of the Law of Ethics of Research of Living Things. This was in line with the recommendation of Alahmad et al15 (with regards to the structure, content and the use of international guideline as reference) in developing the research guidelines.
In conclusion, following the recommendations of Alahmad et al,5 we succeeded in developing research guidelines of international standards with respect to the cultural, traditional, and religious considerations of KSA, and that would provide a valuable framework for the researchers at our institution to conduct investigations with appropriate ethical medical care and that would guide them through vital decision making process.
Acknowledgement
The authors thank Ibn Sina National College for Medical Studies, Jeddah, Kingdom of Saudi Arabia for the initiative to undertake the presented work. We acknowledge the contributions of all the task team members especially Prof. Fathi M. ElGamal for critically reviewing the research guidelines involving human subjects and research data management, Prof. Mohammed ElMoselhy, Prof. Fadia Metwally, and Prof. Ekhalas Nassar for reviewing the section of research involving animal subjects and non-living objects. We thank the external and internal reviewers, Ms. Soumya Ponnan for the draft revisions of the research guidelines, Mr. Abdulqader Almkkawi for the Arabic translation, and Editage for English editing services (https://www.editage.com).
Footnotes
Disclosure. Authors have no conflict of interests, and the work was not supported or funded by any drug company.
- Received October 18, 2018.
- Accepted February 25, 2019.
- Copyright: © Saudi Medical Journal
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.