Abstract
Objectives: To compare the clinical efficiency of the CR220 intraoperative remote assistant device used by the surgical team with that of the custom sound (CS) system used by an audiologist.
Methods: This was a prospective clinical study in a quaternary care center (King Abdullah Ear Specialist Centre) in Riyadh, Saudi Arabia, between October 2018 and March 2019. We included adult and pediatric patients who underwent cochlear implant (CI) surgeries. For every participant, the intraoperative CI testing was performed via both the aforementioned methods. The time taken to complete the measurements with both approaches, including the time required by the audiologist to reach the operating room (OR) and to complete the test, was recorded.
Results: There were no significant differences in the number of responding electrodes between the 2 approaches. For the 25 participants, the time taken for the measurements was 566 minutes with the CS and 173 with the CR220 systems. This significant difference indicates that considerable time can be saved.
Conclusion: The CR220 enables intraoperative CI electrode tests and auto-NRT measurements. Its ergonomics and ease-of-use help the surgical team conduct the tests without an audiologist in the OR, resulting in the efficient use of clinical resources. Further, the results generated were consistent with those of the CS system.
The evaluation of the impedances and neural response thresholds represent important possible tests to be performed during the intraoperative moment. In other words, it is recommended to perform diagnostic tests, including impedance testing and recording the electrically evoked compound action potentials (ECAP) to examine the cochlear implant before the patient leaves the operating room (OR). Impedance is a measurement of the resistance to the electrical current flow. An additional advantage of measuring the impedance is to examine the electrode’s overall function in order to detect potential problems, such as short circuits or breaks in the electrode.1 Recording the ECAP is noninvasive, and it is an objective measurement of the action potential of the cochlear nerve of CI users.2 Further, ECAP threshold measurements are used postoperatively, such as during the audiological programming sessions for the CI system. Measuring neural responses in order to create hearing profiles would be useful for CI candidates belonging to all the age groups; however, it plays a role in the case of pediatric patients and CI users with difficulties in providing subjective feedback .3 In recent times, several technically advanced methodologies have been developed in the field of CI surgery with the following aims: to improve the quality of care, for the proper utilization of medical resources, and to maximize patient satisfaction. The concept of remote intraoperative device testing is one of these developments. With this technique, the goal of measuring device-related parameters can be met without the need for the physical presence of a dedicated audiologist in the OR. A previous study has examined this concept by using a hospital intranet connection between 2 remote laptops, one in the OR connected to the other one in the audiologist’s clinic, and they concluded that this approach could replace the regular intraoperative monitoring while keeping in mind the difficulties that the audiologist might face in travelling from their clinic to the OR, especially if the 2 are located in different buildings.4 On the other hand, the same technical equipment, including specific external devices and a laptop with the software to record measurements, that is used by the audiologist to run tests is present in the OR, which may result in additional work for the operating team who also need to be familiar with the setups and connections involved in using this equipment. Another study utilized remote cochlear testing for the programing process post-implantation and found it to be feasible and useful in terms of saving time.5 Cochlear Limited (Macquarie University, Australia) has integrated the automatic electrical ECAP threshold measurement capability into a novel handheld device with a simpler-to-use interface-the CR220 Intraoperative Remote Assistant that can perform the CI device testing without the requirement for other equipment apart from an external sound processor. The results of a previous study on the first generation of this device (CR120) generated a high degree of confidence in the view that it can replace the standard clinical computer setup for intraoperative CI measurements.6 In our study, we aimed to investigate and understand the levels of increase in clinical efficiency obtained with CR220 compared with the standard clinical system when CR220 is used by the surgical team and when the custom sound (CS) system is used by the audiologist for the intraoperative measurements.
Methods
This prospective study was performed at King Abdullah Ear Specialist Centre (KAESC), Riyadh, Saudi Arabia, between October 2018 and March 2019. This center is considered as one of the quaternary care centres in KSA and one of the leading centre in the Middle East for ear surgery.
The Internal Review Board reviewed and approved our study plan involving the use of human participants with the reference number: 18/0615/IRB. The study performed in accordance with the tenets of the Declaration of Helsinki. Prior to the date of surgery, either our patients or their legal guardians provided informed consent for their participation in our study.
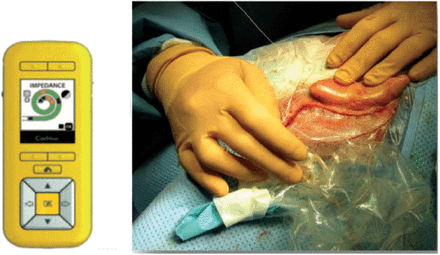
This study was performed for evaluating the efficacy of using 2 of the following types of intraoperative automated testing procedures, both of which were employed in 25 consecutive cochlear implant surgeries: The Remote Assistant (Cochlear® Nucleus CR220) (Figure 1) and the CS system. Our inclusion criteria consisted of children and adults with severe to profound sensorineural hearing loss who underwent cochlear implant surgeries and received Nucleus CI24RE/CI422/CI500 series of implants. Subjects were excluded if there was evidence that they had malformed or ossified cochleae or if, for any reason, the intraoperative measurements could not be completed. The general demographic data of the participants are presented in Table 1.
Remote Assistant (CR220) and its processor with a coil used intra-operatively.
Demographic data of the candidate.
The same surgical technique and steps were implemented for each of the patients. The minimal surgical approach that we used did not require the patients’ hair to be shaved. It involved making a 3 cm surgical incision, followed by performing the drilling process to complete the cortical mastoidectomy and to create a tight periosteal pocket for the receiver. As the surgeon approaches the step involving the drilling of the facial recess, the audiologist is called to attend and begin setting up the equipment to perform the intraoperative measurements. For every candidate, the measurements were recorded twice, once with the CR220 and another time with the CS system. In CS, AutoNRT was measured on all electrodes using an ascending/descending method with a starting current level of 170 (with pulse width of 25 us and stimulation rate of 250 Hz). Due to the ease of using the CR220, this testing procedure was conducted by the surgical team members (who were both familiar with the device and were able to use it). Subsequently, the second measurement was captured by the audiologist using the CS system. The data we collected included the time required to complete each step involved in both measurement processes.
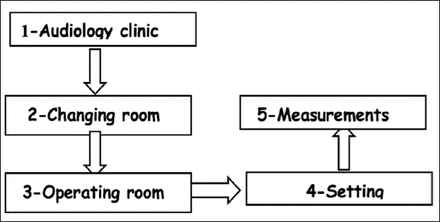
Both testing procedures required a connection to the same external speech processor that was used to communicate with the internal unit and that was covered with a plastic sheet. The number of responding electrodes and the required duration of time were compared for each cochlear implant surgery. The specific details regarding the time measurements associated with the time required by the audiologist to commute from the audiology unit to the OR and to complete the test are presented in Figure 2.
Details regarding the time needed to perform intraoperative CI testing. Time taken to travel from the audiology clinic to the changing room = travelling time, time from the changing room to the operating theatre = entry time to the operating room, time needed to complete the setup to record the measurements = setup time, and the time needed to perform the measurements when the setup was complete = measurement time.
Statistical analysis
We used the statistical package of XLSTAT, version 2018.4, for all the statistical analyses. Data were analyzed to compare the results between the 2 different methods among paired dependent samples using the Wilcoxon test. The results were considered significant at p=0.05
Results
Twenty-five pediatric and adult patients were participated as subjects. The intraoperative CI testing was successfully completed for all participants using both the CS system and CR220. For the first 14 candidates, the measurements were first performed using the CR220, followed by the CS system. It should be noted that (Table 2) when the CS system was used, an increase in the number of responding electrodes was observed for some candidates, and therefore, for the remaining half of the candidates, the measurements were first recorded using the CS system, followed by the CR220. While there is “pre-conditioning” stimulation delivered prior to the ECAP threshold measurements being made, with both systems, it would still appear that there is a stimulation order effect present. This could be attributed to the fact that sometimes air bubbles disappear after the first sweep and/or electrode conditioning that is performed by either CS or CR220.
The measurements associated with the responding electrodes and time (in minutes) durations.
The total time taken to record the measurements for the 25 candidates are presented in Table 2. There were no significant differences between the number of responding electrodes recorded by using the CS system and CR220 (p=0.2202). However, there were significant differences between the time taken to complete the measurements using the CR220 and CS system (p=0.00178). Additionally, there were significant differences between the time duration required to setup the CR220 and CS (p=0.004) system. As a result, there was a significant difference between the total time required to use the CR220 and CS system (p=0.00001).
Discussion
This study investigated the duration of intraoperative testing of cochlear implan to investigate the duration of intraoperative testing of cochlear implants when performed by the surgical team using the CR220. This study has expanded on the results that were reported by Tavartkiladze et al7 whose study was conducted to investigate the equivalency in the performances of the CR220 device and the CS system.
In another recently published study on CR220, Tanamati et al8 compared the intraoperative testing process with CR220 and the CS system. They reached the conclusion that there were no statistical differences associated with the impedance and ECAP values but the time taken to perform the test was significantly lowered when the CR220 was used.8 In a study conducted by Shapiro et al,4 they compared the 2 approaches, remote testing and on-site testing, and they found that the average amount of time the audiologist spent for remote testing was 9 minutes compared to 93 minutes for on-site testing. The aspect they highlighted was the time the audiologist required to complete the test, and they found that in on-site testing, the most significant amount of time was spent during the travel from the audiologist’s clinic to the OR, which was approximately 29 minutes. The time period was shorter in our study with the average being 6 minutes, which was probably due the proximity of the audiology unit in our case. In either case, the elimination of this travelling time is one of the most important advantages of remote testing. The “in-operating-theatre” presence of an experienced audiologist to operate the CS system may not be necessary if well trained operating-theatre staff members were familiar with the testing methods; this could be theoretically applicable, but it may be easier and more practical for surgical team members to use the remote assistant. In our study, the utilization of remote testing by the same surgical team resulted in a significant reduction in the duration of the tests compared to the time required by the audiologist to complete the intraoperative testing. With the increasing the number of implant surgeries required nowadays, this difference could increase progressively, and instead of this time being spent on the intraoperative measurements, it could be utilized to more efficiently serve more patients in the audiology clinic, for example, for programming postoperatively. We encountered some differences in the number of responding electrodes that increased during the second measurement using the CS system for some of the candidates. A potential reason for this could be that during the waiting period for the first measurement, this number could increase owing to the increased period during which the air bubbles could have dissipated; however, this waiting time would have influenced the measurement time between the 2 techniques in this process. Therefore, we opted to document only the first response in order to avoid this. This was noticed in candidates 4, 6, and 14 (Table 2).
The results of this study do not support the replacement of the Custom Sound® EP by the CR220, but they demonstrate an effective and fast alternative for the performance of basic tests during the cochlear implant surgery, in situations where it is not possible to have an audiologist in the operating room. This highlights the limitations of the CR220. Although rare, should the need for different parameters arise (different pulse width, spread of excitation measurements, and so forth), it is unlikely that this could be addressed by the OR staff and an audiologist would be required to present at the OR to use custom sound EP.
- Received October 13, 2019.
- Accepted February 3, 2020.
- Copyright: © Saudi Medical Journal
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.