Abstract
Objectives: To assess the serum concentrations of soluble T-cell immunoglobulin and mucin domain 1 (sTIM-1) and soluble P-selectin (sP-selectin) in individuals who had obstructive sleep apnea-hypopnea syndrome (OSAHS).
Methods: Between December 2020 and November 2022, 134 participants from the Sleep Monitoring Center of the Branch Hospital of Huai’an First People’s Hospital, Jiangsu, China, engaged in this cross-sectional study. Participants were categorized as mild OSAHS (n=19), moderate OSAHS (n=22), severe OSAHS (n=57), and non-OSAHS (n=36) groups. Serum levels of sTIM-1, sP-selectin, and interleukin (IL)-6, as well as baseline clinical characteristics and polysomnography outcomes were assessed in each participant.
Results: Compared to the non-OSAHS group, sTIM-1 and sP-selectin levels were considerably elevated in people who had moderate or severe OSAHS (all p<0.05), but there were no notable changes between those who had mild OSAHS and non-OSAHS participants (p>0.05). The sTIM-1 and sP-selectin levels showed positive associations with the apnea-hypopnea index, body mass index (BMI), and IL-6 levels (all p<0.001). While elevated sTIM-1 was independently related to OSAHS (odds ratio [OR]=1.134, p=0.001), sP-selectin was not associated with OSAHS after adjusting for BMI (OR=1.013, p=0.467).
Conclusion: People with moderate or severe OSAHS had higher serum sTIM-1 and sP-selectin levels, and elevated sTIM-1 is an independently related factor for OSAHS.
The morbidity for people who have obstructive sleep apnea-hypopnea syndrome (OSAHS) is high, ranging from 4% in men to 2% in women.1 Snoring, sleep structural issues, and daytime tiredness are the 3 major clinical symptoms of OSAHS.2 In individuals with OSAHS, intermittent hypoxia results in chronic inflammation, oxidative stress, and increased production of cytokines and adhesion molecules, which may be involved in the mechanism for target organ damage.3 Accumulating evidence supports OSAHS as a low-grade chronic inflammatory disease, associated with immune cell activation and trafficking.4,5
T-cell immunoglobulin and mucin domain-1 (TIM-1), additionally referred to as kidney injury molecule-1, is the first member to be recognized in the TIM family. There are 5 components to TIM-1: a transmembrane region, an IgV-like domain, a mucin-like domain, PS-binding pockets, and an intracellular tail.6 The TIM-1 is primarily expressed on activated CD4+T cells, tubular epithelial cells, various B-cell subsets, natural killer cells, and mast cells, which participates in the process of inflammatory and immunological responses.7 Soluble TIM-1 (sTIM-1) is shed by membrane-proximal cleavage under the hydrolysis of metalloproteinases and circulates in the blood owing to a shortage of the transmembrane region and intracellular tail.8 Inhibition of TIM-1 activity resulted in reduction of leukocyte infiltration in liver models of ischemia/reperfusion injury.9 Circulating TIM-1 levels significantly increase after ischemic kidney injury, and stroke risk increases with elevated plasma TIM-1 levels.10,11 These studies indicate that TIM-1 plays a vital role in hypoxia-related diseases. Therefore, we speculated that TIM-1 might participate in hypoxia during the progression of OSAHS. However, no evidence has been reported on the association between TIM-1 and OSAHS.
The selectin family is categorized as adhesive molecules, comprising L-selectin, E-selectin, and P-selectin. Both the Weibel-Palade bodies of endothelial cells and the alpha-granules of platelets contain P-selectin. P-selectin can be transported to the cell surface within a few minutes after platelets and endothelial cells are stimulated by hypoxia, cytokines, or inflammation.12 When the extracellular domain of P-selectin is spliced, soluble P-selectin (sP-selectin) gets released into the circulation.13 It was unclear from earlier studies whether sP-selectin levels increased in individuals with OSAHS.14,15
In order to find evidence between TIM-1 and OSAHS, we examined the serum levels of sTIM-1 in individuals with OSAHS in this research. Additionally, we also measured the serum concentrations of sP-selectin to determine whether patients with OSAHS have higher sP-selectin levels than healthy controls. Our objective was to search for potential serum biomarkers for OSAHS.
Methods
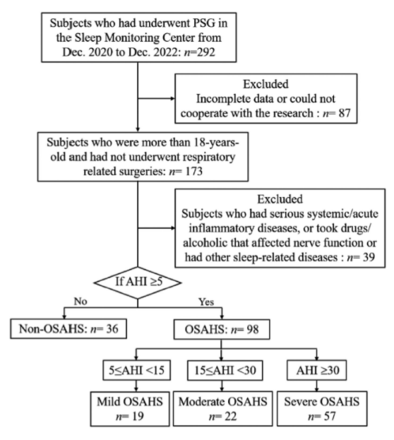
We first enrolled 292 participants in this cross-sectional study who reported snoring and daytime inattention and carried out polysomnography (PSG) at the Sleep Monitoring Center of the Branch Hospital of Huai’an First People’s Hospital in Jiangsu, China, between December 2020 and November 2022. Subsequently, 134 participants who met the criteria were screened (Figure 1). The following were the inclusion requirements: I) age ≥18 years; and II) no prior use of continuous positive airway pressure, a ventilator, or related surgery. The following were the exclusion requirements: I) serious systemic conditions (including acute ischemic stroke, autoimmune disease, and severe cardiac insufficiency); II) acute inflammatory diseases (including upper respiratory infection, pneumonia, pelvic inflammation, and urethritis); III) consumption of alcohol, caffeine, stimulants or sedatives within 24 hours of PSG; IV) other sleep-related conditions; V) lack of informed consent; and VI) incomplete data.
- Flow chart of the enrolling of all participants. OSAHS: obstructive sleep apnea-hypopnea syndrome, PSG: polysomnography, AHI: apnea-hypopnea index
Referring to the guidelines for the diagnosis of OSAHS,16 134 participants were categorized as having mild OSAHS (apnea-hypopnea index [AHI]: ≥5 to <15 events/hour, n=19), moderate OSAHS (AHI: ≥15 to <30 events/hour, n=22), severe OSAHS (AHI: ≥30 events/hour, n=57), and non-OSAHS participants (AHI: <5 events/hours, n=36) (Figure 1).
According to the Expert Agreement on Overweight/Obesity,17 body mass index (BMI) data were transformed into qualitative variables as normal weight (BMI: ≥18.5 to <24 kg/m2), overweight (BMI: ≥ 24 to <28 kg/m2), and obese (BMI: ≥28 kg/m2) in binary logistic regression. The 1964 Helsinki statement and its following amendments, as well as any other comparable ethical norms, guided every procedure carried out during the study involving humans. The informed consent was signed by every participant. The investigation was authorized by the ethics committee of the Branch Hospital of Huai’an First People’s Hospital, Jiangsu, China (ID: HAYYFY2020-KY002).
The hospital information system was used to collect the baseline clinical data for all participants, including age, gender, BMI, history of smoking, hypertension, diabetes, and any other specific diseases.
Participants who did not drink alcohol/caffeinated beverages and were not taking stimulants/sedatives underwent PSG for at least 7 hours using the Philips Alice 4 diagnostic sleep system (Philips Respironics, Commonwealth of Pennsylvania, America). The PSG tests included the AHI, oxygen saturation (SpO2), position, nasal and oral respiratory airflow, pulse, electrocardiogram, electroencephalogram, and electromyogram. All data, including AHI, lowest SpO2 (LSpO2), mean SpO2 (MSpO2), and time percentage for SpO2<90% (SpO2<90%), were collected and stored in a database at our computer center in real-time. The PSG results of all participants were analyzed by otolaryngologists to diagnose OSAHS and evaluate the severity level.
To detect the serum concentrations of sTIM-1 and sP-selectin, we used human sTIM-1 ELISA kit (REF: SY-H04109) and human sP-selectin ELISA kit (REF: SY-H05593) purchased from Shanghai Shuangying Biotechnology Co., Ltd. (Shanghai, China). The serum interleukin (IL)-6 concentrations were measured using a fluorescent immunoanalyzer (Model: AFS-1000), which was bought from Guangzhou Labsim Biotech Co., Ltd. (Guangzhou, China).
Statistical analysis
The Statistical Package for the Social Sciences, version 23.0 for Microsoft Windows (IBM Corp., Armonk, NY, USA) was used to analyze all of the data. Analyzing qualitative variables was carried out using the Pearson Chi-squared test. To ascertain whether quantitative variables had a normal distribution, the Shapiro-Wilk test was utilized. To check for group homogeneity of variance, Levene’s test was applied. For variables having a normally distributed distribution, one-way analysis of variance (ANOVA) was carried out, and the LSD/Tamhane’s T2 test was employed to assess multi-group comparisons. For analyzing variables with non-normal distributions, the Kruskal-Wallis H-test was applied, and the Bonferroni test was used to assess multi-group comparisons. To evaluate the association between 2 variables, the Spearman’s rho test was applied. Investigation of the OSAHS-related factors was carried out using binary logistic regression. While qualitative variables are expressed as numbers and precentages (%), quantitative variables are written as mean ± standard deviation. Differences that met the threshold for statistical significance of 0.05 or less were regarded as significant.
Results
Table 1 displays comparisons of clinical data for the 4 groups. Regarding gender, age, smoking, diabetes, and hypertension history, there were no notable variations among the 4 groups. However, there were significant differences among the 4 groups in terms of BMI, AHI, LSpO2, MSpO2, and SpO2<90%.
- Baseline characteristics of the 4 groups.
The 4 groups differed significantly in terms of sTIM-1, sP-selectin, and IL-6 concentrations, and the multiple comparisons between each group are displayed in Table 2.
- Soluble T-cell immunoglobulin and mucin domain 1, soluble P-selectin, and interleukin-6 levels of the 4 groups.
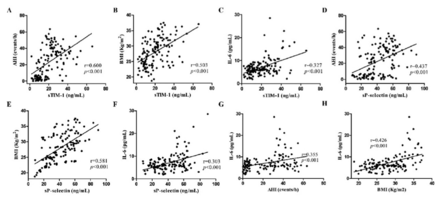
Serum concentrations of sTIM-1 were positive associated with BMI, AHI, and IL-6 values in every participant. The AHI, BMI, and IL-6 values all showed positive correlations with sP-selectin levels. Serum IL-6 values were positively correlated with AHI and BMI levels. All of the correlations were statistically significant (Figure 2).
- Correlations among sTIM-1, sP-selectin, AHI, BMI, and IL-6 values. All results from the 4 groups were included in Spearman’s rho test. AHI: apnea-hypopnea index, BMI: body mass index, sTIM-1: soluble T-cell immunoglobulin and mucin domain 1, sP-selectin: soluble P-selectin, IL-6: interleukin-6, r: Spearman’s correlation coefficient
In Table 3, binary logistic regression (0 = non-OSAHS and 1 = OSAHS) was carried out to analyze independently associated factors for OSAHS to control for confounding factors such as overweight and obesity in patients with OSAHS. Characteristics like gender, age, smoking, diabetes, and hypertension was adjusted in Model 1, and BMI and the factors in Model 1 was adjusted in Model 2. As indicated in Table 3, sTIM-1 and sP-selectin were significant in Model 1. In Model 2, overweight, obesity, and sTIM-1 were significant, whereas sP-selectin was not significant.
- Analysis of related factors using binary logistic regression for obstructive sleep apnea-hypopnea syndrome.
Discussion
Two-thirds of all patients with OSAHS are obese. Every 10% increase in baseline weight was found to cause a 6-fold rise in the risk of having OSASH, which is most likely caused by the build-up of adipose tissue in the neck and belly and changes in upper airway compliance.18 It is important to take comorbid obesity into account when interpreting data on OSAHS since obesity itself might cause systemic inflammation.19
The most notable pathophysiological change in patients with OSAHS caused by chronic intermittent hypoxia is the secretion of adhesion molecules and inflammatory mediators.20 Substantial activation of immune cells in OSAHS leads to a pro-inflammatory cytokine microenvironment. The cytokine IL-6 is released by a variety of circulating blood cells, including macrophages and lymphocytes. The IL-6 is an extensively studied pro-inflammatory cytokines in the pathophysiology of OSAHS.21 Numerous studies have shown that IL-6 levels are considerably higher in individuals with OSAHS than controls.22,23 Here, we discovered that serum concentrations of IL-6 were notably higher in people who had severe OSAHS, which could be attributed to the worse inflammatory environment caused by severe OSAHS. Conversely, several studies did not show a significant difference in serum IL-6 concentrations between individuals who had OSAHS and controls.24 We also found similar results that serum IL-6 levels in the mild or moderate OSAHS groups were not statistically elevated compared to those in the non-OSAHS group. We hypothesized that in individuals who had mild or moderate OSAHS, the production and inhibition of IL-6 maintained a transient balance. The IL-6 levels were positively associated with BMI and AHI levels, and we speculated that obesity and intermittent hypoxia had an impact on the generation of IL-6.
Recent studies reported that T-cells are activated during inflammation or oxidative stress caused by OSAHS.25,26 Activated T-cells are attracted to vascular endothelial cells and migrate to tissues. T-cell activation and trafficking can make the inflammatory environment worse if the stimulus is not removed.27 According to numerous studies, TIM-1 is essential for immune system activation and serves as a co-stimulatory factor for the activation of T-cells.28 Under physiological conditions, TIM-1 is primarily found in the Golgi apparatus, endoplasmic reticulum, and lysosome, and it is found at a very low level on the surface of T-cells. Intracellular TIM-1 moves to the cytoplasmic membrane during T-cell activation, and eventually more TIM-1 proteins were expressed on the cell surface.29 The TIM-1 is correlated with kidney disease, asthma, and diabetes mellitus, and increased circulating TIM-1 levels are associated with the incidence of stroke and progression of kidney injury.30-34 Prior research has suggested an association between TIM-1 and disorders connected to hypoxia. However, the relationship between sTIM-1 and OSAHS has not been investigated in any recent research. The moderate and severe OSAHS groups in this study showed a considerable increase in sTIM-1 values, and there was a positive association between sTIM-1 and AHI values. This suggests that elevated sTIM-1 is associated with the deterioration of hypoxia. The IL-6 and BMI levels both had a positive correlation with sTIM-1 levels, and we hypothesized that BMI or chronic inflammation might affect the generation of sTIM-1. In the logistic regression analysis, sTIM-1 was significantly associated with OSAHS regardless of whether BMI levels were included in the logistic analysis, which demonstrated that elevated sTIM-1 was an independently associated factor for OSAHS. A possible mechanism is that chronic intermittent hypoxia can trigger an inflammatory environment that activates CD4+ T-cells and causes intracellular TIM-1 to be transported to the surface of the CD4+ T-cells. This results in sTIM-1 shedding under the hydrolysis of metalloproteinases and elevated circulating sTIM-1 levels in the blood.
The sP-selectin levels are elevated in conditions involving activated platelets and endothelial cells such as obesity, stroke, and cardiovascular disease.35-37 It is debatable if sP-selectin levels rise in people with OSAHS. In this investigation, the sP-selectin levels in the moderate or severe OSAHS group increased observably than controls and showed a positive correlation with AHI levels, suggesting that they were related to the aggravation of hypoxia. Additionally, sP-selectin levels were strongly linked with BMI and IL-6 levels, implying that abnormal weight or systemic inflammation might account for increased levels of sP-selectin. In the logistic regression analysis, elevated sP-selectin was associated with OSAHS in Model 1. However, the relationship between sP-selectin and OSAHS was not maintained after adjusting for BMI in Model 2. We speculate that comorbid overweight or obesity plays a predominant role in activating platelet or endothelial cells, leading to the shedding and circulation of sP-selectin in people with OSAHS.
Study limitations
Some women patients with OSAHS have atypical clinical manifestations, such as difficulty in falling asleep and insufficient sleep.38 Hence, the percentage of men patients with OSAHS in the clinic was higher than the real percentage in the general population. Possibly for this reason, the percentage of men patients with OSAHS reached 80-95% in this study. The population of this cross-sectional study included those who were suspected of having OSAHS in the clinic and underwent PSG at the Sleep Monitoring Center of Huai’an First People’s Hospital in Jiangsu, China, resulting in a greater proportion of people with OSAHS and a smaller number of non-OSAHS participants. These aspects led to an inevitable sampling bias, and participants in this study were not sufficiently numerous, serving as limitations of the research. In the future, we may recruit more volunteers from the community to increase the number of participants and reduce the sampling bias.
In conclusion, patients with moderate or severe OSAHS have elevated serum levels of sTIM-1 and sP-selectin, and increased sTIM-1 is an independently related factor for OSAHS. These results reveal that serum sTIM-1 may act as a potential serum biomarker, which can help improve the diagnosis and disease monitoring for OSASH.
Acknowledgment
The authors gratefully acknowledge Editage (www.editage.cn) for their English language editing.
Footnotes
Disclosure. This study was supported by Huai’an Natural Science Research Program, Jiangsu, China (HAB202137).
- Received June 20, 2023.
- Accepted September 21, 2023.
- Copyright: © Saudi Medical Journal
This is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.