Abstract
Objectives: To investigated the effects of Bailing capsule on hypoxia-induced proliferation of pulmonary arterial smooth muscle cells (PASMCs).
Methods: This prospective study was performed at the Central Laboratory, Chengdu Medical College, Chengdu, China between April 2012 and November 2014. Ten healthy adult male Wistar rats were administrated with gastric perfusion of Bailing capsule to obtain serum containing the tested drugs. Proliferation of pulmonary arterial smooth muscle cells proliferation was measured using cell counting kit-8 assay. Production of reactive oxygen species (ROS) in rat PASMCs was determined through a fluorometric assay, whereas production of endothelin-1 (ET-1) was detected by ELISA and quantitative real-time PCR (qRT-PCR). Expression of proliferating cell nuclear antigen (PCNA), c-fos, and c-jun in PASMCs was also determined using immunohistochemistry staining and qRT-PCR.
Results: We observed that the medicated serum obviously inhibited hypoxia-induced cell proliferation in a concentration-dependent manner. Moreover, the medicated serum significantly reduced hypoxia-induced production of ROS and ET-1, as well as expression of PCNA, c-fos, and c-jun, in PASMCs.
Conclusion: Results demonstrated that Bailing capsule can inhibit hypoxia-induced PASMC proliferation possibly by suppressing ET-1 and ROS production and by inhibiting expression of PCNA, c-fos, and c-jun. These results suggest that Bailing possess antiproliferative property, which is probably one of the underlying mechanisms of Bailing capsule for the clinical treatment of chronic obstructive pulmonary disease.
Cordyceps sinensis (C. sinensis) is a famous traditional Chinese medicinal fungi demonstrating a wide range of health-promoting and therapeutic functions. Cordyceps sinensis exerts significant immunomodulatory, antioxidant, anti-aging, antiviral, antibacterial, anti-inflammatory, and other effects, as well as demonstrates clinical protective effects on the lung, kidney, central nervous system, immune system, heart, liver, and so forth.1-4 Cordyceps sinensis is a rare and exotic mushroom as it grows all year round on the head of a mummified caterpillar thriving at an elevation of 3000-5000 m above sea level at temperatures below 20°C in the Tibetan Plateau and the Himalayas.5 Production of C. sinensis is thus limited by natural conditions, and this fungus is expensive, thereby limiting the clinical application of C. sinensis. Therefore, an artificial culture of C. sinensis is important. Bailing capsule was first isolated from a culture of C. sinensis obtained from Yushu County and Hualong County of Qinghai Province in China in 1983, and the strain used was Synnematium sinensis (S. sinensis).6 Further study confirmed that S. sinensis is synonymous to Hirsutella sinensis, which is an anamorph of C. sinensis.7 The composition of cultural mycelium of C. sinensis in Bailing capsule are identical to that of wild C. sinensis mainly containing adenosine, mannitol, ergosterol, polysaccharides, a variety of amino acids, vitamins, and trace elements, and thus can be used as a substitute for wild C. sinensis.8 Many studies have shown that Bailing capsule effectively sedates the central nervous system, increases immune function, regulates the endocrine system, exerts anti-inflammatory, anti-hypoxia, and anti-tumor effect, as well as demonstrates an obvious protective effect on the kidney, lung, liver, and so on.5,9 In addition, Bailing capsule exerts an obvious curative effect in patients with chronic obstructive pulmonary disease (COPD).10-12 However, little is known on the pharmacological mechanism by which Bailing capsule exerts its effects against COPD. Progression of COPD can result in hypoxemia, further causing pulmonary vascular structural remodeling, whose key feature is suggested to be the hypoxia-induced abnormal proliferation of pulmonary arterial smooth muscle cells (PASMCs).13-15 The aim of this study is to investigate the effects of Bailing capsule on hypoxia-induced PASMC proliferation and its possible mechanisms to explore the pharmacological mechanism of Bailing capsule in COPD treatment.
Methods
This study was conducted at the Central Laboratory, Chengdu Medical College, Chengdu, China between April 2012 and November 2014. All procedures and protocols were approved by the Laboratory Animal Center of Chengdu Medical College. In addition, this experiment was performed according to the National Guidelines for Care and Use of Laboratory Animals.
Preparation of medicated serum
The Bailing capsules, which mainly contain zymoyic fungal powder of C. sinensis were provided by Hangzhou Chinese-American Huadong Pharmaceutical Company Limited. After removing the shell, the powder contained in Bailing capsule was dissolved in aseptic 0.5% sodium carboxymethylcellulose. A total of 20 rats were randomly divided into Bailing capsule group and control group, each group containing 10 rats. The rats in the Bailing capsule group were administrated via gastric perfusion of 1.5 g/kg Bailing capsule once a day for 7 days, whereas the control group was treated with the same volume of sodium carboxymethylcellulose. Blood was aseptically obtained from the abdominal aorta of the rats 2 hours after the final administration, and the serum was isolated by centrifugation of the blood at 720 × g for 20 min. Following 2 rounds of filtration using a 0.22 µm cellulose acetate membrane, the serum was bottled, calefied in water at 56°C for 30 min, and then stored at -20°C until use.
Primary culture and purity identification of rat PASMCs
Distal pulmonary arteries were isolated from 6-8 week-old Wistar rats, and the endothelium and adventitia were removed carefully by rubbing the luminal surface with a cotton swab by using 0.1% type II collagenase digestion. Small fragments were prepared and placed in 25 cm2 culture dishes in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, L-glutamine (1.6 mM), penicillin G (100 U/mL), and streptomycin sulfate (100 µg/mL), and then maintained at 37 °C in a humidified atmosphere of 95% air and 5% CO2. The PASMCs were passaged at 1:3 ratio until confluence was reached. The morphology of the PASMCs was observed under an inverted microscope, and cell purity was determined using fluorescence immunohistochemistry.
Cell treatment and cell viability assay
Pulmonary arterial smooth muscle cells in passages 6-8 were seeded in cell culture plates at a density of 2 × 105 cells/mL. When 80% confluence was reached, the cells were growth-arrested for 24 hours in a serum-free medium and then divided randomly into 5 groups, each of which was treated with one of the following regimes: 1) normoxia group (nor group), in which the cells were incubated in a complete medium containing 20% normal rat serum under normoxic conditions (5% CO2, 21% O2, and 74% N2); 2) hypoxia group (hyp group), in which the cells were incubated in a complete medium containing 20% normal rat serum under hypoxic conditions (5% CO2, 2% O2, and 93% N2); 3) hypoxia+20% medicated serum group (hyp+20% MS group), in which the cells were incubated in a complete medium containing 20% medicated serum under hypoxic conditions; 4) hypoxia+10% medicated serum group (hyp+10% MS group), in which the cells were incubated in a complete medium containing 10% medicated serum and 10% normal rat serum under hypoxic conditions; and 5) hypoxia+5% medicated serum group (hyp+5% MS group), in which the cells were incubated in a complete medium containing 5% medicated serum and 15% normal rat serum under hypoxic conditions. Trypan blue exclusion test was conducted to evaluate the cytotoxic effect of drug on PASMCs and to detect cell viability. The results showed that different concentrations of serum containing the tested drugs did not exert any toxicity on PASMCs. Moreover, the cell survival rate was more than 95%.
Cell proliferation assay
The PASMCs of each group were seeded into 96-well plates containing 200 µL of complete medium, and 3 replicates were prepared per group. After 48 hours incubation, 10 µL of cell counting kit-8 (CCK-8) was added into each culture and then incubated for 2 hours. The absorbance was measured at 450 nm and then referenced at 630 nm by using a microplate reader.
Measurement of reactive oxygen species (ROS) production
Reactive oxygen species production in PASMCs of each group was determined via a fluorometric assay (GenMed Scientifics Inc., Wilmington, USA) by using dihydroethidium bromide (DHE) as probe for the presence of ROS. After pre-incubation for 24 hours, the PASMCs of each group were incubated with DHE for 20 min. The fluorescence intensity was measured at 540 nm excitation and 590 nm emission using a fluorescence microplate reader (BioTek Instruments, Inc, USA).
After the cells in each group were cultured for 24 hours, enzyme-linked immunosorbent assay was performed to detect endothelin-1 (ET-1) in the culture supernatant of PASMCs of each group according to the manufacturer’s guidelines (Cusabio Biotech Co., Ltd, USA).
Cell immunohistochemistry
For proliferating cell nuclear antigen (PCNA), c-fos (provide the full meaning?), and c-jun (provide the full meaning?) immunohistochemical assays, the PASMCs in each group were grown on microscope slides for 24 hours, fixed in 4% paraformaldehyde solution, and pretreated with 0.5% Triton X-100 in phosphate buffer saline(PBS). Immunostaining was performed as described previously. The anti-rat c-jun, c-fos, and PCNA antibodies were purchased from Santa Cruz, USA. The expression levels of c-fos and c-jun were quantified using an automated image analysis system (Image-Pro Plus 5.0, Media Cybernetics, Inc., Bethesda, USA). The positively stained area was measured in at least 10 high-power fields (400× magnification). In addition, PCNA expression was quantified by calculating the percentage of positively stained cells in each high-power field.
Quantitative real-time PCR (qRT-PCR)
After the cells in each group were cultured for 12 hours, the total RNA was extracted using RNAprep pure cell/bacteria kit according to the manufacturer’s instructions. The total RNA was reverse transcribed into cDNA using ReverTra Ace qPCR RT kit (Toyobo, Osaka, Japan) according to the manufacturer’s instructions. The primers used for qRT-PCR were designed according to the mRNA sequences of the target genes in GenBank databases. The primer sequences purchased from Invitrogen are listed in Table 1. Moreover, each cDNA sample was amplified in triplicate using Real Master Mix (EVAGreen™). The relative amounts of mRNA of the target genes were also normalized to that of β-actin.
Primer sequences for real-time polymerase chain reaction.
Statistical analysis
The values were presented as mean ± standard error of mean. Multiple group means were compared using one-way analysis of variance, and q test was used for pairwise comparisons. Data on rate and enumeration were analyzed using x2 tests. All values were considered significant at p<0.05.
Results
Purity identification of cultured PASMCs
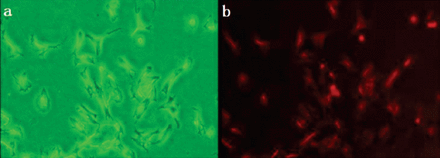
The rat PASMCs in culture were spindle-shaped and contain centrally located nuclei. The cells grew in multiple layers and exhibited a typical morphology of “valley and hill” after confluence. Fluorescence immunohistochemical staining with primary antibodies against rat alpha-smooth muscle actin revealed that more than 97% of the cells emitted red Cy3 fluorescence under a fluorescence microscope (Olympus, Tokyo, Japan) (Figure 1).
Identification of rat pulmonary arterial smooth muscle cells (PASMCs). Isolated PASMCs were fixed and subjected to immunofluorescence with specific alpha smooth muscle actin (α-SMA) antibody. A) The cells were observed under the optical microscope; B) The red-stained myofilaments observed under the fluorescence microscope represented the positive antigen of α-SMA (400×magnifications).
Effect of Bailing capsule on hypoxia-induced PASMC proliferation
Cell proliferation was measured through CCK-8 assay. Hypoxia significantly induced PASMC proliferation compared with normoxia. However, the hypoxia-induced PASMC proliferation was obviously suppressed by medicated serum in a concentration-dependent manner as revealed by the significantly lower cell viability in PASMCs culture treated with both 20% and 10% medicated serum than in PASMCs of the hypoxia group (Figure 2).
Bailing capsule inhibits hypoxia-induced proliferation of rat pulmonary arterial smooth muscle cells. *p<0.05, **p<0.01 compared with the hyp group. hyp group - hypoxia group, hyp+20%MS - hypoxia + 20% medicated serum, hyp+10%MS - hypoxia + 10% medicated serum, hyp+5%MS - hypoxia + 5% medicated serum
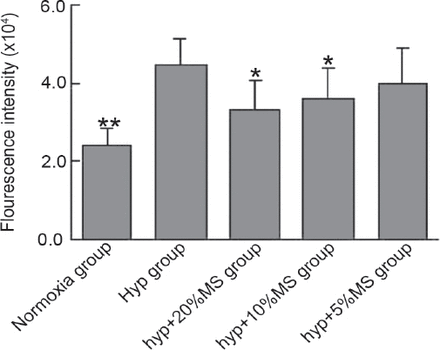
Bailing capsule inhibits hypoxia-induced reactive oxygen species production in rat pulmonary arterial smooth muscle cells. *p<0.05, **p<0.01 compared with the hyp group. hyp group - hypoxia group, hyp+20%MS - hypoxia + 20% medicated serum, hyp+10%MS - hypoxia + 10% medicated serum, hyp+5%MS - hypoxia + 5% medicated serum
Effect of Bailing capsule on hypoxia-induced ROS production in PASMCs
Compared with that under normoxic condition, exposure to hypoxia for 24 hours obviously increased ROS production in PASMCs as indicated by increased fluorescence intensity, whereas 20% and 10% medicated serum both significantly reduced the elevated amount of ROS. These results demonstrated that Bailing capsule can prevent ROS production in PASMCs under hypoxic condition.
Effect of Bailing capsule on hypoxia-induced ET-1 production in PASMCs
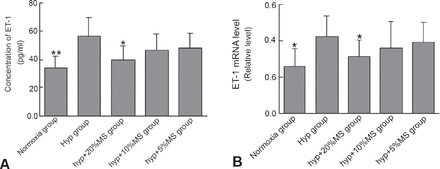
Enzyme-linked immunosorbent assay analysis showed that ET-1 contents in supernatant of PASMCs cultured under hypoxic condition were significantly increased compared with that under normoxic condition. However, the 20% medicated serum significantly inhibited the release of hypoxia-induced ET-1 from PASMCs (Figure 4A). To evaluate the effect of Bailing capsule on ET-1 gene expression, we isolated the total cellular RNA from PASMCs and analyzed the PASMCs via qRT-PCR using ET-1 primer. The results showed that mRNA expression of ET-1 was significantly higher in the hypoxia group than in the normoxia group. Moreover, medicated serum significantly reduced the hypoxia-induced ET-1 mRNA expression in a concentration-dependent manner (Figure 4B).
Bailing capsule inhibits hypoxia-induced endothelin-1 (ET-1) production in rat pulmonary arterial smooth muscle cells (PASMCs). A) enzyme-linked immunosorbent assay (ELISA) detects ET-1 in the culture supernatant of PASMCs of each group. B) ET-1 a messenger RNA (mRNA) expression in rat PASMCs in each group. *p<0.05, **p<0.01 compared with the hypoxia group.
Effect of Bailing capsule on hypoxia-induced PCNA, c-fos, and c-jun expression in PASMCs
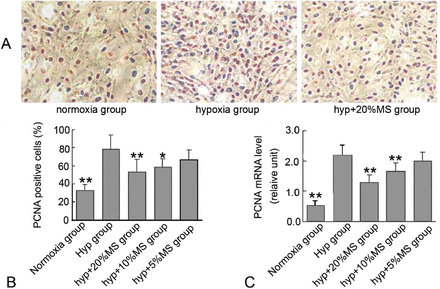
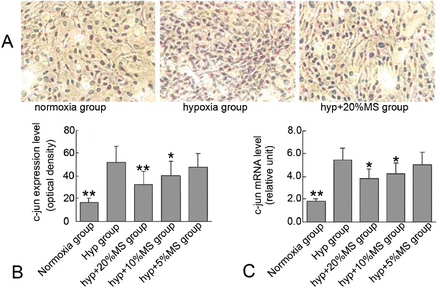
We examined the effect of Bailing capsule on hypoxia-induced PCNA, c-fos, and c-jun expression in PASMCs to investigate the possible mechanism associated with the inhibitory effect of Bailing capsule on hypoxia-induced VSMC proliferation. Cell immunohistochemistry revealed that hypoxia significantly induced intracellular expression of PCNA, c-fos, and c-jun compared with that in the control group, whereas medicated serum reduced hypoxia-induced PCNA, c-fos, and c-jun expression in a concentration-dependent manner (Figures 5 & 7).
Bailing capsule inhibits hypoxia-induced proliferating cell nuclear antigen (PCNA) expression in rat pulmonary arterial smooth muscle cells (PASMCs). A) Representative immune staining for PCNA expression in PASMCs in culture. B) Quantification of PCNA expression by calculating the percentage of positive stained cells. C) PCNA mRNA expression in PASMCs in each group. *p<0.05, **p<0.01 compared with the hypoxia group.
Bailing capsule inhibits hypoxia-induced c-fos expression in rat PASMCs. (A) Representative immune staining for c-fos expression in PASMCs in culture. (B) Quantification of c-fos expression by using an automated image analysis system. (C) c-fos mRNA expression in PASMCs in each group. *p<0.05, **p<0.01 compared with the hyp group.
Bailing capsule inhibits hypoxia-induced c-jun expression in rat pulmonary arterial smooth muscle cells (PASMCs). A) Representative immune staining for c-jun expression in PASMCs in culture. B) Quantification of c-jun expression by using an automated image analysis system. C) c-jun messenger RNA (mRNA) expression in PASMCs in each group. *p<0.05, **p<0.01 compared with the hyp group.
Total cellular RNA was also isolated from PASMCs and was analyzed via qRT-PCR using PCNA, c-fos, and c-jun specific primers to confirm the possibility that Bailing capsule inhibits PCNA, c-fos, and c-jun expression by regulating their mRNA levels. The qRT-PCR results showed that the mRNA expression levels of PCNA, c-fos, and c-jun were significantly higher in the hypoxia control group than in the normoxia group. In addition, the medicated serum significantly reduced hypoxia-induced PCNA, c-fos, and c-jun mRNA expression in a concentration-dependent manner (Figure 8A), and the degree of mRNA inhibition was comparable with that of intracellular protein expression.
Discussion
Bailing capsule contains zymoyic fungal powder of C. sinensis produced via the microbial submerged liquid culture method, and its pharmacological action is basically consistent with that of wild C. sinensis. Bailing capsule obviously demonstrates a curative effect in COPD patients by invigorating the lung, enhancing immunity, anti-inflammation, and anti-hypoxic effect, by inhibiting airway remodeling, and so on.5 Chronic obstructive pulmonary disease is a major cause of mortality and morbidity worldwide and is often complicated by development of hypoxia-induced pulmonary arterial hypertension (HPH), which is a serious disorder characterized by pulmonary vasoconstriction and enhanced proliferation of PASMCs that lead to structural remodeling of blood vessel walls.16 Hypoxia, inflammation, and other elements play important roles in the development of HPH.17
This study detected the effect of Bailing capsule on proliferation of rat PASMCs under hypoxic conditions. We found that the medicated serum significantly reduced hypoxia-induced PASMC proliferation. By testing the effect of different drug concentrations, this study found that the cell survival rate was more than 95%. Therefore, we can rule out the possibility that the inhibitory effect of Bailing capsule on PASMC proliferation resulted from drug toxicity.
The mechanism of action of antiproliferative agents may involve their effects on cell cycle regulatory proteins. Proliferating cell nuclear antigen is a non-histone nuclear protein and is widely used as “proliferation marker” both in its normal and diseased states; PCNA is expressed mainly in the S-phase of the cell cycle, and this finding is consistent with the proliferation results.18 We investigated the effect of medicated serum on hypoxia-induced PCNA expression in PASMCs to understand the possible mechanism associated with the inhibitory effect of Bailing capsule on cell proliferation. The results indicated that medicated serum inhibited PCNA expression at the protein and mRNA levels in a concentration-dependent manner, suggesting that the inhibitory effect of Bailing capsule on hypoxia-induced PASMC proliferation can be mediated by inhibiting PCNA expression.
Increasing oxidative stress augments HPH, whereas decreasing oxidative stress reverses HPH, suggesting that oxidative stress is implicated in HPH development.19 Agents promoting ROS generation stimulate proliferation of both systemic arterial smooth muscle cells and PASMC.20 Moreover, suppression of endogenous ROS production inhibits smooth muscle cell proliferation.21 The present study found that under hypoxic condition, medicated serum significantly inhibited ROS generation in PASMCs, suggesting that inhibition of ROS production renders the protective effects of Bailing capsule against PASMC proliferation.
Endothelin-1, a potent endothelium-derived vasoconstrictor and is abundant in pulmonary vasculature, has been implicated in the development of pulmonary hypertension.22 Endothelin-1 is also produced by other cells, such as leukocytes, macrophages, and smooth muscle cells, which are involved in vascular disease.23 In the lung, ET-1 expression is increased by hypoxia. In addition to its vasoconstrictive effect, ET-1 demonstrates mitogenic effect on PASMCs.24 This study found that under hypoxic condition, medicated serum significantly inhibited ET-1 generation in PASMCs. Thus, we can speculate that the inhibitory effect of Bailing capsule on hypoxia-induced PASMC proliferation involves inhibition of ET-1 expression.
The cellular genes c-fos and c-jun dimerize to form the activator protein-1 transcription factor complex, which upregulates transcription of a diverse range of genes.25 These genes are involved in proliferation, differentiation, and in a series of pathophysiological processes.26 Furthermore, c-jun and c-fos are both induced under hypoxic conditions and may increase proliferation rate of PASMCs.27 We found that treatment with medicated serum significantly inhibited the expression levels of c-jun and c-fos in PASMCs cultured under hypoxic conditions, suggesting that Bailing capsule inhibits hypoxia-induced cell proliferation in PASMCs possibly by suppressing expression of the transcription factors c-jun and c-fos.
In conclusion, our results demonstrate that Bailing capsule can inhibit hypoxia-induced PASMC proliferation possibly by suppression of ET-1 and ROS production and by inhibition of PCNA, c-fos, and c-jun expression. These data suggest that Bailing capsule possesses antiproliferative property, which is probably one of the underlying mechanisms of Bailing capsule for the clinical treatment of COPD.
Footnotes
Disclosure. Authors have no conflict of interests, and the work was not supported or funded by any drug company. This work was supported by grants from Sichuan Science and Technology Support Program, China (Grant No. 30504010109)
- Received January 31, 2016.
- Accepted February 26, 2016.
- Copyright: © Saudi Medical Journal
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.