Abstract
Objectives: To highlight the clinical benefit, efficacy, and safety of zoledronic acid (ZA) therapy in children and adolescents with primary and secondary osteoporosis.
Methods: This is a retrospective observational study of 131 children and adolescents visiting the Pediatric Endocrine Clinic at King Abdulaziz University Hospital, Jeddah, Kingdom of Saudi Arabia, between January 2002 and January 2015. Clinical and laboratory data were collected for each patient and adverse events were evaluated.
Results: The mean patient age was 11.43 years. There was a significant decrease in the number of fractures after ZA treatment for primary osteoporosis (p=0.000) and in secondary osteoporosis (p=0.005). There was a significant decrease in both osteocalcin (p=0.001) and C-terminal telopeptide (p=0.003) in patients with primary osteoporosis, as well as osteocalcin (p=0.003) and C-terminal telopeptide (p=0.008) in patients with secondary osteoporosis after treatment.
Conclusion: The use of ZA in children and adolescent appears to have favorable effects on fracture rate and quality of life, including pain and mobility in symptomatic individuals. Intravenous ZA is comparable to other bisphosphonate agents in its efficacy and safety and features a more convenient infusion protocol with no documented long-term complications, thus, we advise its use in pediatric population.
Once a concern exclusively in the elderly, osteoporosis is becoming a major concern among the global pediatric population.1 Despite being the most common human metabolic disorder in adults, its prevalence in the pediatric population is not well identified. Osteoporosis is generally characterized by a decrease in bone mass and density that can lead to an increased fracture susceptibility. As childhood and adolescence are critical periods for skeletal maturity, as well as bone growth and strength, osteoporosis can hamper those parameters in various ways. Therefore, teenagers should be targeted as an at-risk population, and preventive measures should be implemented to maximize bone mass, and theoretically decrease lifelong fracture risk. Causes of childhood osteoporosis are classified into primary; those that are due to an intrinsic bone abnormality (usually genetic in origin), and secondary; those that are due to underlying medical conditions, or their treatment. Both primary and secondary osteoporosis have been observed and considered in this study population. Bisphosphonates are pyrophosphate analogs that increase bone mineral density (BMD) by inhibiting bone resorption, which favors bone formation during remodeling.2,3 They are currently the main pharmacological agents for the management of childhood osteoporosis.4 Zoledronic acid (ZA; Zometa Inc., Novartis Pharma AG, Lichtstrasse, Switzerland) is a new-generation heterocyclic nitrogen-containing bisphosphonate that has demonstrated markedly higher potency, and a greater therapeutic ratio in clinical trials than earlier generation bisphosphonates, including pamidronate.5,6 Up to date, many studies have investigated bisphosphonate treatment primarily with the use of pamidronate in many bone related diseases.7 As to the ZA treatment of pediatric osteoporosis, there are no published data on long-term use, safety and efficacy. Moreover, there are no Saudi local, Arabian, or even internationally published data on a large study number of children receiving ZA. In this study, we aim to review a 13-year experience with primary and secondary causes of osteoporosis, as well as the efficacy, and safety of intravenous ZA as the treatment of choice in our pediatric population at the King Abdulaziz University Hospital (KAUH), Jeddah, Kingdom of Saudi Arabia (KSA).
Methods
This retrospective observational study examined children and adolescents with primary and secondary osteoporosis followed up at the Pediatric Endocrine Outpatient Clinic at KAUH, Jeddah, KSA between January 2002 and January 2015. A total of 131 patients aged 6 weeks to 18 years were included in the study. Data were obtained from a direct interview of patients and/or their parents, and all laboratory results were obtained from the KAUH electronic Phoenix system. These data comprised clinicodemographic information, anthropometric measurements, and main etiological factors and treatment methods. Informed verbal consent was acquired from all patients and/or their parents prior to the start of therapy. Patients with clinical or biochemical (high bone turnover marker, C-terminal telopeptide [CTX], and osteocalcin levels) confirmed the diagnosis of osteoporosis, and/or a z-score ≤-2.0 SD on a bone densitometry (DXA) scan were included in the study. Moreover, patients with a normal bone profile (calcium, phosphate, and alkaline phosphatase), as well as normal total vitamin D and parathyroid hormone (PTH) levels before the start of treatment were included. Exclusion criteria included mineral metabolism disturbances, major data insufficiency, and a creatinine clearance rate <30-35 mL/min.
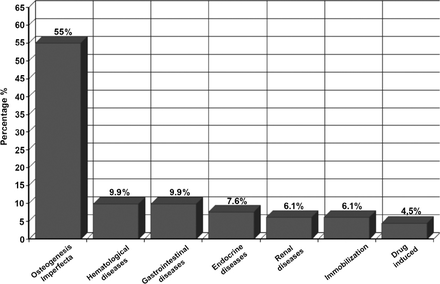
We studied 2 groups of subjects. Group one included (72/131) patients diagnosed with primary osteoporosis. Group 2 comprised (59/131) patients with secondary osteoporosis, which was sub-classified into 6 categories according to disease etiology (Figure 1). Intravenous ZA was used as the treatment of choice in both groups throughout this research-based study. Zoledronic acid was administered intravenously at a dose of 0.05 mg/kg (maximum dose to be given is 2 mg/infusion) over 60 minutes. In neonates and infants, the dose was 0.025 mg/kg. The first 5 infusions were given once every 3 months, then once every 6 months, depending on the clinical and biochemical marker response. All patients were admitted to the general ward for 2 days to receive their first infusion to enable close monitoring of the acute complications that might occur. Acute complications of the first dose were fever, myalgia, hypocalcemia, flu-like symptoms, and bone pain. To prevent hypocalcemia, all patients were given a continuous intravenous calcium infusion of 200-400 mg/kg/day. In addition, ibuprofen 10 mg/kg was administered 3-4 times per day to minimize the fever and myalgia that were frequently observed in patients after the first infusion. Subsequent ZA infusions were given during day care unit admissions. All patients were advised to maintain sufficient oral calcium intake consisting of a daily dose of 1200 mg together with a daily dose of prophylactic vitamin D (400-800 IU).
Sub-classifications of main disease etiology of patients with secondary osteoporosis at King Abdulaziz University Hospital.
Statistical analysis
The data analysis was performed using IBM SPSS Statistics version 20.0 software (IBM Corp., Armonk, NY, USA). Continuous variables are expressed as mean ± standard deviation (SD), while categorical variables are shown as percentages. A 2-tailed paired-sample t-test was used to assess the clinical and biochemical efficacy of the drug with a 95% confidence interval. Values of p<0.05 were considered statistically significant for individual variables. Two main search engines were used to find prior related research, Google Scholar and PubMed database of Maastricht University.
Assessments
Clinical Assessment
Prior to treatment and at each subsequent visit, patients and/or their parents were provided with a questionnaire form and asked to note the symptoms they have had experienced before and after starting intravenous ZA treatment. The symptoms described in the questionnaire included pain (generalized, lower back, hip, neck, and/or upper or lower extremities), and the estimated frequency of pain (daily, weekly defined as pain more than one day per week, or infrequently defined as less than one day per week). Body height and weight were measured for all patients in the study. For children aged 2-18 years, body height and weight were converted to age- and gender-standardized scores with SD according to Children’s Hospital Boston Growth Calculator 2.018 since short stature is a feature associated with osteogenesis imperfecta (OI).
Laboratory assessment
Serum calcium, 25-hydroxyl (OH), vitamin D, phosphate, PTH, and alkaline phosphatase levels were measured before the start of treatment. Moreover, creatinine level was calculated at baseline, and at each treatment visit before the ZA infusion. According to measurements at our institute, the normal range of serum calcium is 2.12-2.52 mmol/L, 25-hydroxyl (OH) vitamin D was 50-80 nmol/L, serum osteocalcin was 11-43 ng/mL in females, and 24-70 ng/mL in males, serum CTX was <0.57 ng/mL in females and <0.58 ng/mL in males. Levels of both bone markers (serum osteocalcin and CTX) were measured at baseline, before treatment, and then every 3-6 months throughout the treatment period.
Radiological assessment
Data available on our system for BMD measurements were insufficient to analyze since the hospital’s DXA scan machine had no software supported to measure BMD in patients <5 years of age and frequently malfunctioned. However, many of our patients underwent their initial BMD measurements at facilities other than the selected hospital. Data from our institute’s system were obtained only for 57/131 (31.6%) patients who had their BMD measured before treatment, and 18/57 (13.7%) underwent the measurements after starting their treatment course for comparison. In all patients, total body and lumbar spine BMD measurement z-scores were adjusted for age, gender, puberty, and body size as appropriate. Low BMD was defined as a BMD z-score ≤-2.0 SD. A z-score of -1.0 to -2.0 SD was defined as osteopenia. Eventually, due to lack of sufficient follow-up DXA measurement data, BMD measurements were excluded from the subsequent statistical analysis.
Fracture assessment
The fracture rate per year was calculated by dividing the number of fractured bones prior to the start of treatment by the number of years from the first fracture to the first dose.8 For those in which treatment started before one year of age, fracture rate was calculated by dividing the number of fractures by the duration in months, and then multiplying it by 12. Quality of life (QOL) was also considered and defined as normal, or below normal compared with that in children their age and according to post-treatment improvement.
Adverse events
Acute and chronic adverse events were observed and monitored throughout the study. Acute side effects were reported after the first ZA infusion and included fever, hypocalcemia, decreased intake, bone pain, myalgia, and flu-like symptoms. Nephrocalcinosis was assessed by renal ultrasound at baseline before the start of treatment, and then annually. The renal profile at baseline and after each infusion cycle was checked for any complications.
Results
The study included 131 patients (mean age, 11.43 ± 4.2 years; 48.9% males, 51.1% females) followed-up at KAUH. We studied 2 groups of subjects; group one (72/131; 54.96%) with primary osteoporosis, and group 2 (59/131; 45.03%) with secondary osteoporosis. In our population, the most common form of primary osteoporosis was OI (55.7%). In contrast, the most common causes of secondary osteoporosis in our population were hematological diseases and gastrointestinal diseases, followed by endocrine diseases, renal diseases, immobilization, and drug-induced osteoporosis (Figure 1).
Group one (primary osteoporosis)
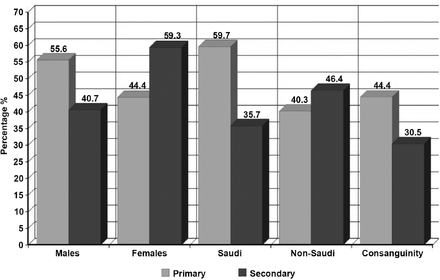
The primary osteoporosis group included 72 children and adolescents (mean age, 9.65 ± 5.40 years; 55.6% males, 44.4% females). There were 43 Saudi (59.7%) and 29 non-Saudi (40.3%) patients of Arabic or Middle-Eastern origin (Figure 2). Mean weight was 26.30 ± 19.34 kg, while the mean height was 110.02 ± 25.59 cm. The mean age at the time of diagnosis was 3.26 ± 4.37 years, mean age at treatment was 4.29 ± 4.02 years, and mean number of doses were 16.34 ± 11.07. Consanguinity was reported in 32/72 (44.4%) patients: of them, 71.9% were first-degree cousins, and 28.1% were second-degree cousins. A family history of osteoporosis <50 years of age was present in 22/72 (30.6%) patients. A bone deformity was present in 43/72 (59.7%) patients before the start of treatment. Fractures were a major clinical characteristic of this group. Fractures were the initial presentation in 53/72 (73.6%) patients, which represents more than 2-thirds of the group’s subjects. The mean number of fractures before treatment was 4.86 ± 8.10, which significantly decreased after treatment to 1.47 ± 5.10 (t(43) = 4.850, p=0.000).
Patient demographics of primary and secondary osteoparosis at King Abdulaziz University Hospital, Jeddah, Kingdom of Saudi Arabia.
Regarding QOL; 40/72 (55.6%) patients were assessed as having below normal QOL prior to treatment. After treatment with ZA infusion, 38/40 (95%) patients reported improved QOL and 2/40 (5%) patients reported no change. A 2-tailed paired-sample t-test revealed a significant subjective improvement in QOL (t(52) = 6.385, p=0.001) with a confidence interval (CI) of 95%. Concerning biochemical results, the mean pre-treatment levels were as follows: calcium 2.296 ± 0.18 mmol/L; phosphate 1.157 ± 0.20; alkaline phosphatase (ALP) 155.49 ± 88.81 IU/L; PTH 2.04 ± 1.25 pg/mL; and vitamin D 108.1 ± 46.28 nmol/L. The mean osteocalcin pre-treatment value was 91.03 ± 53.48, and post-treatment value was 60.78 ± 28.25 ng/mL, while the mean pre-treatment value was 0.74 ± 0.72, and post-treatment CTX value was 0.42 ± 0.31 ng/mL. There was a significant decrease in both osteocalcin (p=0.001) and CTX levels after treatment (p=0.003).
Group 2 (Secondary osteoporosis)
The secondary osteoporosis group included 59 children and adolescents (mean age 14.76 ± 4.64; 40.7% males, 59.3% females): 33 (35.7%) Saudi and 26 (46.4%) non-Saudi of Arabic or Middle-Eastern origin (Figure 2). Mean weight of the population was 31.54 ± 18.45 kg while the mean height was 124.34 ± 25.14 cm. Mean age at diagnosis was 10.90 ± 4.49, mean age at start of treatment was 11.21 ± 4.53, and mean number of doses was 7.25 ± 8.37. Consanguinity was reported in 18/59 (30.5%) of the study group: 66.7% of them were first-degree cousins and 33.3% were second-degree cousins. A family history of osteoporosis <50 years was reported in 7/59 (11.9%) patients. Bone deformity was present in 10/59 (16.9%) of the study subjects. Regarding fractures, 10/59 (16.9%) patients presented with fractures with a mean pre-treatment number of 0.34 ± 0.73, which showed statistically significant improvement after treatment to 0.01 ± 0.04 (t(37) = 2.96, p=0.005).
Concerning QOL, 17/59 (28.8%) patients subjectively reported a below normal QOL. After treatment, 11/17 (64.9%) patients reported improved QOL, while 5/17 (29.4%) patients reported no change in their QOL. Regarding biochemical results, mean calcium level before treatment was 2.22 ± 0.17 mmol/L, mean phosphate level was 0.98 ± 0.41, mean ALP level was 132.69 ± 98.7 IU/L, mean PTH was 3.23 ± 2 pg/mL, and mean vitamin D level was 77.33 ± 23.75 nmol/L. The mean pre-treatment was 72.52 ± 46.96, and post-treatment osteocalcin value was 39.53 ± 23.33 ng/mL, On the other hand, the mean pre-treatment CTX level was 1.08 ± 0.72, and post-treatment CTX level was 0.44 ± 0.24 ng/mL. Thus, there was a significant post-treatment decrease in both osteocalcin (p=0.003) and CTX levels (p=0.008).
Clinical safety
Data of both groups were included in the efficacy and safety analyses. An acute-phase reaction, including fever, hypocalcemia, flu-like symptoms, decreased intake, and bone pain usually occurs in most children with the initiation of intravenous or oral agents. Of the 72 studied patients with primary osteoporosis (OI), 28 (38.9%) had fever, 19 (26.4%) had flu-like symptoms, 17 (23.6%) had a decreased intake, and 14 (19.4%) had bone pain. Using a paired t-test, we found a statistically significant improvement in pain frequency after ZA treatment (t (17) = 4.994, p=0.000, 95% CI). In contrast, of the 59 patients in the secondary osteoporosis group, 11 (18.6%) had fever, 8 (13.6%) had hypocalcemia, 6 (10.2%) had flu-like symptoms, 6 (10.2%) had decreased intake, and 28 (47.5%) had bone pain. Using the same paired t-test, evidence proved a statistically significant improvement in pain frequency post-treatment in the secondary group (t(18) = 4.53, p=0.000, 95% CI). Another acute adverse event of ZA infusion was the decrease in calcium level. The mean pre-treatment calcium level in group one was 2.296 ± 0.18 and post-treatment calcium level was 2.149 ± 0.129, while the mean pre-treatment calcium level was 2.22 ± 0.17 and mean post-treatment calcium level was 2.01 ± 0.25 in group 2. This decrease in calcium level was observed during the first ZA infusion. No chronic events were reported throughout our 13-year experience with ZA.
Discussion
The use of bisphosphonates in children with osteoporosis is now well established, and has generally been well tolerated.9 This article summarizes our 13-year experience using ZA therapy in a pediatric population with osteoporosis at KAUH, Jeddah, KSA. Considering the relatively wide study group, this study is considered the first reported long-term observational clinical trial of a Middle-Eastern pediatric population. The management and prognosis of osteoporosis in children are affected by the underlying etiology. According to the present retrospective study, OI was the most prevalent primary etiology presented to our pediatric endocrine clinic. On the other hand, several forms of secondary osteoporosis were prevalent as well (Figure 1).
Since the main goals of pharmacological therapy in osteoporosis, including decreasing the fracture rate, decreasing bone pain, increasing mobility, increasing independence, and decreasing bone turnover marker levels were achieved, the results of this study prove that cyclic intravenous ZA is an efficient treatment for children and adolescents with osteoporosis. In our patient cohort, clinical symptoms improved dramatically after the start of ZA treatment. Fractures and bone pain were the 2 dominant presenting symptoms in our population. We had an encouraging result regarding pain relief and a reduction in fractures after ZA treatment.
Reviewing previously reported studies with other bisphosphonates, a well-documented study of 30 children with OI by Glorieux et al10 showed comparable results with the use of pamidronate. An improved fracture rate accompanied by reduced chronic pain is consistent with the findings of all bisphosphonate therapy to date.11,12 In this study, we noted improved QOL. These results corroborate the conclusions of Batch et al13 that pamidronate therapy undoubtedly improved the QOL and medical morbidity (fracture rate, pain, and immobility) of many children with symptomatic osteoporosis, particularly those with OI and fibrous bone dysplasia. Levels of bone turnover markers osteocalcin and CTX, which reflect osteoclast and osteoblast activity, were higher than normal at baseline. A significant reduction in both CTX and osteocalcin occurred after the start of the ZA infusions in our study group, which verifies the suppression of bone resorption.14 Short-term side effects of ZA infusion are common and consist of acute phase reaction flu-like symptoms, fever, bone pain, and decreased oral intake. Among them, fever was the most common acute side effect in association with bone pain, followed by a decrease in oral intake, and then flu-like symptoms. In another study of ZA use,15 transient flu-like symptoms with bone pain were the most common adverse effects. These reactions occurred mostly 24-48 hours after the first treatment. Such symptoms might be ameliorated by the prophylactic use of paracetamol, or ibuprofen. Subsequent ZA infusions are not usually associated with acute reactions, and the bone pain is attenuated.
Mild-to-moderate hypocalcemia was encountered in 25 patients (19.1%) after the first infusion with no clinical symptoms, and none of our patients demonstrated an acute decrease in phosphate level. However, Hogler et al16 noticed a higher frequency in first dose post-infusion hypocalcemia (74%) and hypophosphatemia (82%) with the use of the same dose of ZA as reported here. There were no chronic adverse events of major concern reported in our cohort of patients, for example, nephro-, gastrointestinal, or ocular toxicities as reported in other studies of the use of oral bisphosphonates, or intravenous pamidronate and alendronate.17,18 A study for Simm et al19 who reported the use of ZA in 20 children with secondary osteoporosis for 2 years supported our findings. This study has examined the adverse effect of ZA treatment on anthropometric parameters and other possible transient side effect. Comparable conclusion was drawn to the safety and efficacy of ZA treatment in a relatively smaller study subject. Although many studies showed that long-term treatment with bisphosphonate is relatively safe, some have stated that it might be absorbed into the bone and can remain there for up to 10 years.20 Although this study achieved its aim, there were unavoidable limitations as mentioned previously with regard to the hospital’s dysfunctional DXA scanner. Molecular screening for genetic mutations was also a limitation since it was not available at our institute.
In conclusion, pediatric osteoporosis is an alarming growing health problem affecting children and adolescents; as such, a high index of awareness should be raised among pediatricians worldwide. Our 13-year experience investigating the efficacy of ZA infusions for improving pediatric osteoporosis clinically and biochemically, as well as examining its short- and long-term safety shows that ZA use should be considered strongly for treatment of children and adolescents with primary or secondary osteoporosis. Children and adolescents with symptomatic osteoporosis (bone pain, recurrent fractures, and impaired mobility) should be considered candidates for ZA therapy. All patients starting on ZA infusion must be given a continuous prophylaxis infusion of calcium, antipyretics, and analgesia to reduce the risk of these deficits.
Corrections, retractions and “Expressions of Concern”
Excerpts from the Uniform Requirements for Manuscripts Submitted to Biomedical Journals updated November 2003. Available from www.icmje.org
The corrections should appear on a numbered page, be listed in the contents page, include the complete original citation and link to the original article and vice versa if online.
The retraction and expression of concern, appear on a numbered page in a prominent section of the print journal as well as in the online version, be listed in the contents page, and include in its heading the title of the original article. The text of the retraction should explain why the article is being retracted and include a full original citation reference to it.
Editors may ask the author’s institution to assure them of the validity of earlier work published in their journals or to retract it. If this is not done editors may choose to publish an announcement expressing concern that the validity of previously published work is uncertain.
Footnotes
Disclosure. Authors have no conflict of interest, and the work was not supported or funded by any drug company.
- Received June 11, 2015.
- Accepted September 22, 2015.
- Copyright: © Saudi Medical Journal
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.