Abstract
Inflammatory periodontal disease is a major cause of loss of tooth-supporting structures. Novel approaches for regeneration of periodontal apparatus is an area of intensive research. Periodontal tissue engineering implies the use of appropriate regenerative cells, delivered through a suitable scaffold, and guided through signaling molecules. Dental pulp stem cells have been used in an increasing number of studies in dental tissue engineering. Those cells show mesenchymal (stromal) stem cell-like properties including self-renewal and multilineage differentiation potentials, aside from their relative accessibility and pleasant handling properties. The purpose of this article is to review the biological principles of periodontal tissue engineering, along with the challenges facing the development of a consistent and clinically relevant tissue regeneration platform. This article includes an updated review on dental pulp stem cells and their applications in periodontal regeneration, in combination with different scaffolds and growth factors.
Chronic inflammation and loss of the tooth-supporting structures are distinct features of chronic periodontal diseases. Preservation and enhancement of the regeneration of periodontal structures are the main goals of periodontal treatment. However, the periodontium is a complex structure as it contains a minimum of 6 distinct tissue types including: the gingival epithelium, the gingival connective tissue, the periodontal ligament, the tooth root surface cementum, the alveolar bone and the corresponding vasculature. All these tissues are affected during chronic inflammation and restoration of their normal status is important for allowing periodontal regeneration to occur.1,2 Different periodontal surgical procedures concerning root conditioning, autografts, allografts, xenografts, and/or barrier membranes for guided tissue regeneration have been employed to enhance periodontal tissue regeneration.3 While histological evidence of tissue regeneration has been observed in some studies of regenerative therapies, complete periodontal tissue regeneration is still difficult to obtain.4-6 In a previous study,7 we provided adequate description on tissue engineering and the involvement of mesenchymal (stromal) stem cell (MSC) with and without scaffold. Briefly, tissue engineering represents a novel approach for regeneration of damaged tissues and organs. Tissue engineering is based on establishing the essential conditions that support the natural regenerative potential of tissues, and where each functional stage of reconstruction is based on a biologically enhanced process. By employing the conceptual framework of tissue engineering, it may be possible to obtain complete periodontal tissue regeneration. The purpose of this article is to review the biological principles of periodontal tissue engineering, along with the challenges facing the development of a consistent and clinically relevant tissue regeneration platform.
Components of periodontal tissue engineering
The essential components of periodontal tissue engineering are a) cells including stem cells, b) scaffold materials, and c) appropriate signals like morphogens/growth factors. Each one of these components plays an important role in the regenerative process. The cells define the nature of the tissue to be regenerated, morphogens and growth factors are required to direct the proliferation and the differentiation of cells to specific tissue fate, and scaffolds are used to provide a 3-dimensional micro-environment to facilitate 3-dimensional-tissue formation and enhancement of lineage differentiation. These 3 components are the focus of studies of periodontal tissue engineering.8
Stem cells
Stem cells are defined as undifferentiated cells that exhibit self-renewal and multi-lineage differentiation capacity. Stem cells can be classified into pluripotent (embryonic) or induced-pluripotent stem cells, and adult (also known as tissue-specific) stem cells.7,9 Recently, a number of adult stem cell types have been isolated from dental tissues, including dental pulp stem cells (DPSCs),10-13 exfoliated deciduous teeth (SHED),14-16 periodontal ligament (PDLSCs),17,18 apical papilla (SCAP),19-21 and dental follicle progenitor cells (DFPCs).22,23 In addition, putative stem cells have been isolated from inflamed pulpal,24,25 and periodontal26 tissues.
Dental pulp progenitor cells are the most attractive cells for periodontal tissue engineering based on their good growth and differentiation capacity in ex vivo cultures. Dental pulp progenitor cells are derived from mesodermal tissues and have been originally described by Gronthos et al.11 They are closely related to mesenchymal (stromal) stem cells (MSC) that are present in the stromal compartment of different tissues including bone marrow. A Gene expression profile of DPSCs has been reported to be similar to that of bone marrow MSC.27 Those cells are derived from embryonic neural crest cells and exhibit self-renewal and multilineage differentiation potentials.10,11,28,29 Dental pulp progenitor cells can differentiate into a number of mesodermal and non-mesodermal tissue cells that include osteoblast,13,28,30 adipocytestes,28,29 chondrocytes,28,29 and myocytes,28,29 as well as neuronal,28,31 and endothelial cells,30,32 hepatocytes,33 melanocytes,34 in addition to the dentin forming odontoblasts.35 It is not known whether DPSC cultures contain a homogenous population with respect to differentiation or contain subpopulations with different lineage specific differentiation potentials. In support of the later hypothesis, CD34+ subpopulations of DPSCs has been reported to be committed to bone formation evidenced by formation of mineralized nodules and bone tissue.36,37 Further subpopulations with different characteristics are also being studied.38,39 Overall, DPSC represent a unique cell population with potential for dental tissue engineering.
Isolation and characterization of DPSC
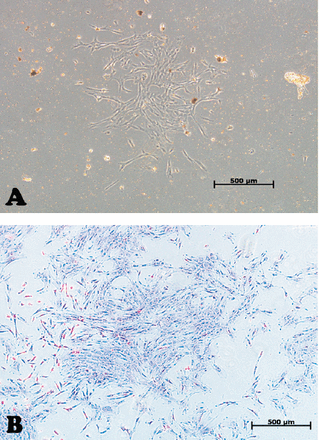
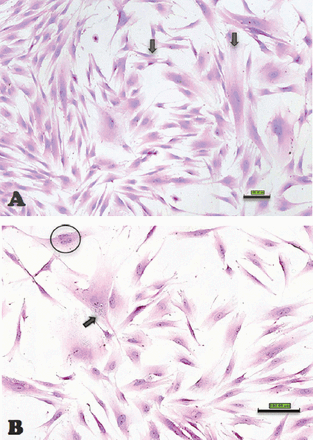
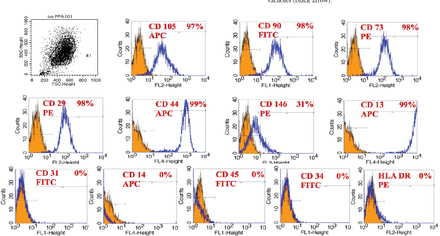
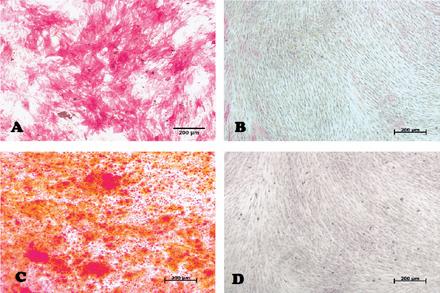
In our laboratory, we established DPSCs, which were isolated from the pulp tissue of extracted human third molar teeth. The teeth were vital, free from caries or periodontal diseases, or infections. Pulp tissue was exposed to enzymatic digestion and resultant cells were cultured in standard medium. Dental pulp stem cells initially formed multiple colonies similar to the MSC-derived colony forming unit fibroblast (CFU-F) (Figure 1). The cells exhibit heterogeneity in size and shape. They contained pale, round or oval central nucleus with multiple nucleoli, which indicated active DNA transcription and RNA synthesis. Multiple cytoplasmic vacuoles represented cellular secretory vesicles (Figure 2). These morphological characteristics are similar to MSC obtained from bone marrow and other tissues.11,40 Similar to MSC, DPSCs were positive to CD 105, CD 90, CD 70 and showed weak or negative expression of the common hematopoietic markers CD 34, CD 45, CD 31, CD 14, and HLA-DR.41 (Figure 3). Dental pulp progenitor cells differentiated readily into osteoblast-like cells in vitro as shown by presence of alkaline phosphatase activity and ability to form mineralized matrix (Figure 4). When implanted in vivo (subcutaneously) in immune deficient mice/rabbits, DPSCs formed dentin-pulp-like complex.10,42 These characterizations and differentiation capacities were confirmed with different tissues in our laboratory.
Inverted light microscopic images showing: A) Primary culture of dental pulp stem cells. B) The morphology of a dental pulp stem cells’ colony passage 2. Original magnification 5x. Scale bar 500µm
Scanoscope images representing different morphologic features of dental pulp stem cells (scale bar 1 mm) A) Cells with difference in size (black arrows) B) A multinucleated cell (nucleus circled with black), and a cell with cytoplasmic vacuoles (black arrow).
Fluorescence activated cell sorting (FACS) analysis results of a representative dental pulp stem cell line.
Inverted light microscopic image representing dental pulp stem cells 14 days post osteogenic induction after staining with: Alkaline phosphatase (ALP) A) test (red color represent ALP) B) control, and alizarin red-S (ARS) C) test (red color represent mineralized nodules), and d) control original magnification 10x, scale bar 200µm.
Scaffolds
For achieving periodontal regeneration, scaffolds are required to guide the 3-dimensional growth of tissues. Scaffolds should be able to support cell growth and preferably contain growth factors needed for induction of cell differentiation. In addition, the scaffold should be able to permit the transportation of oxygen, nutrients and waste products and should be bio-degreadable to be replaced by the periodontal tissues and should be highly porous with suitable pore size that support the above mentioned functions.43
Scaffold materials
Several scaffolds are currently available for periodontal tissue regeneration. Scaffolds are usually classified as either biologically derived polymers isolated from the extra-cellular matrix of animal tissues, from plants, or seaweed; for example, collagen type I or fibronectin, alginate from brown algae, chitosan from shells, and coralline materials, or synthetic; for example, hydroxyapatite (HA), tri-calcium phosphate (TCP) ceramics, polylactide and polyglycolide and a combination of these in the form of poly DL-lactic-co-glycolic acid (PLGA).44 Several examples have been employed in periodontal tissue regeneration. Biocorals are among the famous natural scaffolds used for that purpose.45 In addition to being resorbable and biocompatible, those scaffolds have interconnected porous structure that enhance cellular seeding, bone ingrowth, and withstand stresses.46 Bone grafts and bone substitutes supply a structure for the development of the regenerative tissues.47-49 Autologous platelet-rich plasma (PRP) and platelet rich fibrin (PRF) are easy to obtain during the surgical procedure and are rich in bone stimulating growth factors. In addition, platelet concentrates are slowly degradable and can provide a 3D-fibrin matrix formation that can enhance regeneration.50-52 Another scaffold used in periodontal regeneration is mineral trioxide aggregate (MTA),53,54 which is composed of dicalcium and tricalcium silicate, tricalcium aluminate, gypsum, tetra-calcium-alumino-ferrite, and bismuth oxide.55 Mineral trioxide aggregate is biocompatible and exhibit osteoconductive effects on bone cells.56 Tani-Ishii et al57 reported that, in osteoblasts, faster growth of cells and increased mineralized matrix gene expression has been observed in the presence of MTA. Also, Perinpanayagam & Al-Rabeah58 have reported that MTA supports cell attachment and expression of Runt-related transcription factor 2 (RUNX2), the osteoblast lineage master transcriptional factor. In addition, some studies have evaluated the use of different titanium alloys combined with implant surfaces bio-modifications to function as stem cell carrier for enhancement of osteointegration.59,60
Morphogen/growth factors
Growth factors and morphogens are employed to enhance proliferation and differentiation of stem cells into specific tissue type and to stimulate stem cells to synthesize and secrete mineralized matrix. A variety of growth factors have successfully been identified for periodontal regeneration. Enamel matrix derivative (EMD) has been reported to support periodontal regeneration.61,62 It contains a mixture of low molecular weight proteins that get absorbed into hydroxyapatite and collagen fibers of the root surface and induce cementum formation and periodontal regeneration.63 On cellular level, EMD exerts stimulatory effects on periodontal cells.63-66 Other growth factors known to enhance periodontal regeneration have also been employed.67,68 An example is platelet-derived growth factor (PDGF), which is a strong regulatory growth factor in bone that stimulates differentiation of osteoprogenitor cells and enhance vascularization.69 Histological studies showed significant increase in bone and cementum formation upon PDGF treatment.70,71 On cellular level, PDGF increases the number of collagen-synthesizing cells,72 and stimulates cell maturation evidenced by increased bone sialoprotein transcription.73 Another example is bone morphogenetic proteins (BMPs). They were discovered in bone matrix based on their ability for osteoinduction.74 Bone morphogenetic proteins are family of differentiation factors and each is capable of inducing the formation of new bone tissue when administered in vivo. Bone morphogenetic proteins produced by osteoblast, stored in bone matrix and released from bone matrix by osteoclastic bone resorption. The content and diffusibility of BMPs mediate the enhanced bone formation effects of demineralized bone extract when implanted in soft tissues or in osseous tissues.75 Blum et al,76 Pietrzack et al,77 and Wildemann et al78 evaluated the concentration of BMPs in demineralized free-dried bone allografts (DFDBA), and found it to vary with donor age and the source of bone extract, and associated their concentration with osteoinductive ability of the graft.
Combination of bone grafts with growth factors results in efficient tissue regeneration.79 Recombinant human (rh) BMP-2 added to the DFDBA has resulted in increased osteoinduction by almost 50%.80 Jones et al81 reported a higher percentage of defect fill and new bone contact at implant sites treated with rhBMP-2. In addition to the newly formed bone and new cementum, a connective tissue attachment was formed demonstrating the ability of rhBMP-2 application to induce periodontal tissue regeneration.82,83
Another approach involves the use of different histone deacetylase (HDAC) inhibitors to enhance cellular osteogenic differentiation. An example of such material is valporic acid. Although Schroeder and Westendorf84 reported the material to enhance osteogenic maturation, Paino et al85 reported such material may enhance early differentiation events, without inducing terminal differentiation stages.
Examples of periodontal tissue engineering
In cell-based replacement of a functional periodontium, the challenge is to form a physiological attachment between the dental apparatus and the hosting bone, with a cushion effect that absorb and distribute the mechanical load during function. A novel approach for periodontal tissue regeneration is to employ different populations of dental stem cells in order to recapitulate key events during periodontal tissue development; thus, allowing healing to occur in a synchronized way to regenerate the periodontium.86,87 As a corollary, the ideal positioning of cells in a tissue engineering context would include insertion of osteoblastic cells to maintain and repair bone together with periodontal ligament cells supporting a network of vascular and nerve cells. A conceptually simpler approach to periodontal regeneration methods involves engineered cell sheets to facilitate human periodontal ligament cell transplantation together with bone grafting materials and growth factors to regenerate complete periodontal tissues.88,89 However, maintaning the ability of reconstituted periodontium to preserve integrity and function during mastication over long periods of time is currently an area of intensive investigation.
Since alveolar bone loss is a major complication of chronic periodontal diseases, the use of DPSCs for alveolar bone regeneration is clinically relevant. D’Aquino et al,90 implanted DPSCs enriched collagen sponge at the extraction defect of third molar teeth. Radiographic evaluation revealed formation of significant amount of new well organized lamellar bone that was histologically verified at 3 months follow up. There was also more attachment gain next to the adjacent second molar. Interestingly, after 3 years, the regenerated tissue formed complete compact bone.91 Brunelli et al92 tried to utilize DPSCs to perform sinus elevation, and reported successful results as more bone formed during 4 months follow up.
More advanced protocols have demonstrated possible periodontal and craniofacial tissue engineering using different combinations of MSC-like cells from dental and non-dental origins together in combination with growth factors.93-95 Yamada et al,93,94 achieved significant bone regeneration around dental implants using DPSCs combined with platelet rich plasma (PRP) compared to PRP alone and similar results have been reported by Ito et al.95 At experimental levels, a more complex approach has been described by Liu et al96 where the group have reported a successful use of DPSCs in a functionalized hydroxyapatite/collagen/poly (L-lactide) (nHAC/PLA) scaffold using rhBMP-2; to reconstruct critical-size alveolar bone defects in rabbits. The feasibility of utilization of these cell lines in human remains to be determined.
Is whole tooth regeneration possible?
Tooth-like tissues have been generated by seeding a number of cell types on biodegradable scaffolds. The simplest described technique has been to harvest, expand and differentiate cells in vitro, followed by seeding them onto scaffolds, and finally implanting them in vivo. In some cases, the scaffolds were re-implanted into an extracted tooth socket or in the jaw. Ikeda et al97 have successfully regenerated and implanted a fully functioning tooth in an adult mouse, through transplantation of bioengineered tooth germ cells into the alveolar bone of the lost tooth region. The bioengineered tooth was fully erupted and exhibited normal tooth structure, hard-mineralized tissues and adequate response to noxious stimulations such as mechanical stress and pain. Xu et al98 reported seeding rat tooth bud cells on scaffolds fabricated from silk fibroin with 2 different pore sizes. The scaffolds were used as fabricated or after functionalization with an integerin Arg-Gly-Asp (RGD) binding peptide. The scaffolds were placed in the omenta of immune deficient athymic adult rats for 20 weeks. Histological analysis of tissue formed in both scaffolds revealed the presence of mineralized osteodentin-like tissues. Although most dental tissues were regenerated, the success rate was only 15-20% for achieving the correct arrangement of a natural tooth. Further studies are required to achieve replicable reconstituted and structurally sound teeth suitable for human use.
Future perspectives
Despite the impressive progress in tissue engineering approaches for enhancing the regeneration of periodontal tissues, several challenges remain to be solved. Although several animal studies were conducted, more human trials are required to evaluate the efficacy of those procedures in regenerating true periodontal defects. There is a need to evaluate the ability of those transplanted cells to survive, function and integrate into the recipient site in the presence of all other cellular and molecular components, including inflammatory mediators. In preparation for further clinical applications, emphasis on development of clear cell handling protocols to reach good manufacturing practice (GMP) is needed.46 This practice aims at standardization of the cells used in tissue engineering studies, as well as enhancing cell proliferation and differentiation. The production of vascularized scaffolds is a key requirement for enhancing tissue growth.43 Technologies for functionalization of scaffolds with specific signaling peptides that enhance angiogenesis are being developed and tested for their ability for mediating efficient periodontal tissue engineering. Finally, comprehensive interdisciplinary research programs that include cell biologist, material scientists and clinicians are prerequisite for the development of successful and clinically suitable regenerative periodontal tissues.
Copyright
Whenever a manuscript contains material (tables, figures, etc.) which is protected by copyright (previously published), it is the obligation of the author to obtain written permission from the holder of the copyright (usually the publisher) to reproduce the material in Saudi Medical Journal. This also applies if the material is the authors own work. Please submit copies of the material from the source in which it was first published.
Footnotes
Disclosure. This work was supported by the National Plan for Sciences and Technology Program, King Saud University, Riyadh, Kingdom of Saudi Arabia (Grant (No. 10-BIO1304-02).
- Received July 9, 2015.
- Accepted September 22, 2015.
- Copyright: © Saudi Medical Journal
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.