Abstract
Objectives: To demonstrate the pattern of disease-modifying antirheumatic drugs (DMARDs) use in Saudi and non-Saudi rheumatoid arthritis (RA) patients, and to evaluate the association of DMARDs use with anti-mutated citrullinated vimentin (anti-MCV) positivity and other factors.
Methods: Retrospectively, for a period of 7 years (2007-2014), we studied 205 RA patients, at King Abdulaziz University Hospital (KAUH), Jeddah, Saudi Arabia. All patients used DMARDs. Pattern of use for all 6 DMARDs was almost the same among Saudis and non-Saudis with no significant difference (p>0.05) for each DMARD; MTX was the most commonly used DMARD (71-76%).
Results: There was no association between anti-MCV positivity and different DMARDs use. Methotrexate was used 76 times as combination, scoring the highest in this respect. There was a significant correlation (p<0.05) between Plaquenil with Methotrexate and with Sulfasalazine; Leflunomide with anti-TNF and with Prednisolone; age with Methotrexate and with Plaquenil; anti-MCV positivity with Prednisolone. Saudi/non-Saudi status showed no correlation with all factors or drugs. There was no significant association between DMARDs and comorbidity.
Conclusion: Similar to worldwide results, MTX was the most commonly used DMARD; with the addition of anti-TNF to increase the effect, and folic acid to minimize the side effects. In this cohort, the pattern of use for all DMARDs was similar among Saudis and non-Saudis; treatment depended neither on anti-MCV positivity nor on the presence of comorbid conditions. A study of the association of DMARDs with disease activity is recommended.
The effective treatment of rheumatoid arthritis (RA) can be achieved by disease-modifying antirheumatic drugs (DMARDs) that decrease joint damage with improvement of symptoms and functional abilities.1 The DMARDs have been classified into synthetic (sDMARDs) and biological.2 The sDMARDs are traditional drugs; such as methotrexate (MTX), sulfasalazine, leflunomide, and hydroxychloroquine (Plaquenil).2 The sDMARDs also include synthetic glucocorticoids (such as Prednisolone).3 If an sDMARDs is not effective after a trial of 3 months,4 they are usually combined with a biological DMARD, such as tumor necrosis factor alpha (TNF-α) blockers.1 To achieve disease remission in approximately 50 of people and improved overall outcomes, the DMARDs should be started very early in the disease.5 The frequently used DMARDs include MTX (the most commonly used one), Plaquenil (hydroxychloroquine), Azulfidine (sulfasalazine), and Arava (leflunomide), either as monotherapy, or in combination.1 Methotrexate is the most commonly used DMARD wordwide,6,7 and is the first line of treatment;8-10 even according to the treatment guidelines from the American College of Rheumatology (2012),11 and the European League Against Rheumatism (2010).12 Methotrexate is usually combined with folic acid (a vitamin),13 in order to reduce its adverse effects including nausea, vomiting or abdominal pain (gastrointestinal), hematologic, pulmonary, and hepatic.10 Methotrexate is teratogenic, thus, pregnancy should be avoided.8,10 Prednisolone (a synthetic glucocorticoids) can be used in the short term, while waiting for slow-onset drugs to take effect,1 and also as an injections into individual joints.1 Although its long-term use reduces joint damage, it also results in osteoporosis and susceptibility to infections, and thus is not recommended.1 A better effect can be achieved by combining MTX with anti-TNF than with MTX monotherapy.14 The response rate is better when switching from MTX monotherapy to MTX plus anti-TNF than combined DMARDs to MTX plus anti-TNF.14 In this study, our aim was to determine the pattern of disease modifying antirheumatic drugs use, and their association with anti-mutated citrullinated vimentin antibody (anti-MCV) in rheumatoid arthritis patients.
Methods
Data was obtained by retrospective file review for a period of 7 years (2007-2014), at King Abdulaziz University Hospital, Jeddah, Saudi Arabia. During this period, 280 RA patients met the American College of Rheumatology (ACR) 1987 classification criteria15 for RA; files of 205 patients contained complete data for our objectives, including anti-MCV, rheumatoid factor (RF), age, gender, nationality, and treatment regimens; thereby they were included in this study. Anti-MCV was measured by ELISA (Alegria machine from Orgentec Diagnostika GmbH, Mainz, Germany) and was considered positive when the concentration was >20 IU/ml (normal value 0-20 IU/ml). Rheumatoid factor was measured by nephelometry (Behring BN2 Nephelometer, Siemens, Erlangen, Germany) and was considered positive when the concentration was >20 IU/ml (normal value 0-20 IU/ml). All of the 250 patients received DMARDs as soon as the diagnosis of RA was made. Data concerning comorbid conditions was available for 191 patients. The recorded conditions included the following conditions (as single and/or mixed): vitamin D deficiency (VDD), hypertension (HT), hypothyroidism, systemic lupus erythematosus (SLE), diabetes mellitus (DM), rheumatic heart disease (RHD), osteoporosis (OP), epilepsy, and others. The collected data was part of a retrospective review, thus informed consent was not obtained. However, written ethical approval was obtained before commencing the study, and was presented to the filing department before the retrospective review. Entrez-PubMed, Advanced search - PubMed - NCBI, and Saudi Digital Library (SDL) were used for checking references and prior related research.
Statistical analysis
The data was analyzed using the Statistical Package for Social Science (SPSS Inc., Chicago, IL, USA), version 14. The results were illustrated in table and figure format showing comparisons and frequencies of variables. Results were considered significant if the p-value was less than 0.05. An association was analyzed using the odds ratio, and R2 was obtained using scattering blot.
Results
The demographic and clinical characteristics of the RA patients (205 Saudis and non-Saudis) are shown in Table 1. The 205 patients comprised 136 Saudis and 69 non-Saudis. There were 90.7% females and 9.3% males; with a mean age was 46 years (SD=14.23). The prevalence of rheumatoid factor was higher in Saudis than non-Saudis, with no significant difference between the 2 groups. The prevalence of mutated citrullinated vimentin antibodies (anti-MCV) was slightly higher in Saudis than non-Saudis. The ESR and CRP were also higher in Saudis than non-Saudis, with no significant differences for both ESR and CRP.
Demographic and clinical characteristics of 205 Saudi and non-Saudi rheumatoid arthritis patients.
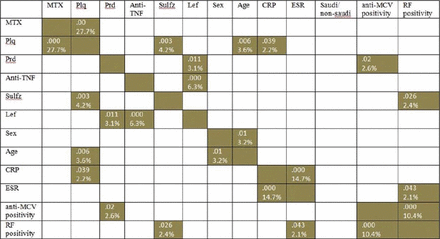
Table 2 shows the frequency of DMARDs treatment among Saudis, non-Saudis, and the total cohort, and their association with anti-MCV positivity. All patients started using DMARDs when the diagnosis of RA was made, and the pattern of use for all DMARDs was similar among Saudis and non-Saudis and the cohort (Table 2). Six DMARDs were used in both Saudis and non-Saudis, with no significant difference between these 2 populations for each DMARD; the most frequently used DMARD was MTX followed by hydroxychloroquine sulphate (Plaquenil), Glucocorticoids (Prednisolone), Adalimumab (HUMIRA), Sulfasalazine and Leflunomide (Arava) (Table 2). A significant correlation between anti-MCV positivity and DMARDs was found with Prednisolone (in the cohort), and with Prednisolone and Plaquenil (among Saudis) (Table 2). In combination with DMARDs, almost one third of the patients used other drugs, with folic acid being the most frequent (Table 2). Details of combined DMARDs treatment in the total cohort is presented in Table 3. Methotrexate was used 76 times as combination scoring the highest in this respect, followed by Prednisolone, Plaquenil, and anti-TNF, Sulfasalazine, and, Leflunomide. The highest combination was between MTX with Plaquenil Prednisolone, followed by MTX with Prednisolone Plaquenil, and MTX with anti-TNF, then Plaquenil with Prednisolone and Leflunomide, then other combinations (Table 3). Table 3 also demonstrates that there were 2 groups of the 88 combined DMARDs use; with MTX [76 (86.5%)] and without MTX [12 (13.5%)]. Methotrexate and Plaquenil, each was equally distributed between single and combination groups. While anti-TNF and prednisolone, each was used 9 times more as a combination than as single; Sulfasalazine and Leflunomide were used only as combination (Table 3). Combined Plaquenil was use 38 times, divided as 26 with MTX and 12 without MTX (Table 3); indicating that 1/3 of Plaquenil combined use was without MTX and 2/3 was with MTX, with a significant correlation (P=0.00) between MTX and Plaquenil (Figure 1). Likewise, frequency of combined MTX was 76 times, divided as 26 with Plaquenil and 50 without Plaquenil (Table 3). Also, 85% of Plaquenil combined use (32/38) was without Sulfasalazine, 15% was with Sulfasalazine (Table 3), with a significant correlation (p=0.003) between Sulfasalazine and Plaquenil (Figure 1). Detailed combinations of the other DMARDs are clearly demonstrated in Table 3. Dark boxes in Figure 1 represent the significant correlation between the 6 different DMARs (MTX, Plaquenil, sulfasalazine, anti-TNF, Prednisolone, leflunomide) with each other and with other factors (gender, age, ESR, CRP, Saudi/non-Saudi status, anti-MCV positivity, and RF positivity). A significant correlation among DMARDs can be classified into 2 groups: MTX, Plaquenil, Sulfasalazine, and Prednisolone, Leflunomide, anti-TNF. In the first group, there was a significant correlation between MTX with Plaquenil (p=0.00), and between Plaquenil with Sulfasalazine (p=003). In the second group, there was a significant correlation between Leflunomide with Prednisolone (p=0.011) and between Leflunomide with anti-TNF (p=0.00). There was a significant correlation between DMARDs and other factors; anti-MCV positivity, and RF positivity) anti-MCV positivity showed a correlation with Prednisolone (p=0.02) and with RF positivity (p=0.00); RF positivity with Sulfasalazine (p=0.026) and ESR (p=0.043); ESR with CRP (p=0.000); age with Plaquenil (p=0.006) and with gender (p=0.01). Saudi/non-Saudi status showed no correlation with any of the other factors or drugs (Figure 1).
Frequency of disease-modifying antirheumatic drugs (DMARDs) use and their association with anti-mutated citrullinated vimentin (anti-MCV) positivity.
Frequency and detailed combined disease-modifying anti-rheumatic drugs (DMARDs) treatment in the cohort.
Correlation of different disease-modifying anti-rheumatic drugs (DMARDs) and other factors with each other. Dark boxes [with p-values (top) and R2 (%)] indicate significant correlation. Anti-MCV - anti-mutated citrullinated vimentin, RA - rheumatoid arthritis, RF - rheumatoid factor, CRP - C-reactive protein, ESR - erythrocyte sedimentation rate, MTX - methotrexate, Plq - plaquenil, Prd - Prednisolone, HUM - hydroxychloroquine sulphate (Plaquenil), Glucocorticoids (Prednisolone), Adalimumab, Sulfz - Sulfasalazine, Lefl - Leflunomide, TNF - tumor necrosis factor. Significant correlation (p<0.05) between Plaquenil with MTX and with Sulfasalazine; Leflunomide with anti-TNF and with Prednisolone; age with Plaquenil and with gender; anti-MCV positivity with Prednisolone and with RF positivity; and CRP with Plaquenil and with ESR. Saudi/non-Saudi status showed no correlation with all factors or drugs
Concerning the correlation between age groups (10 year groupings) and different DMARDs use (not illustrated), age groups showed a significant correlation only with Plaquenil (p=0.008). However, all DMARDs that were used in considerable frequencies [MTX (153 times), Plaquenil (70 times), Prednisolone (60 times) and anti-TNF (22 times)] showed a peak pattern at 40-49 years. While Sulfasalazine (7 times) was used 3 times at 30-39 years, 2 times at 50-59 years, one time at both 40-49 years, and 60-69 years; and Leflunomide (5 times) was used 2 times at the age group 60-69 years, and one time at each of the age groups 20-29, 40-49, and 50-59 years.
Comorbid conditions data was available in 191 patients. Comorbidity was present in 30% of the cohort, 38.5% in Saudis, 48.5% in non-Saudis, with no significant differences between Saudis and non-Saudis for presence of comorbid conditions (Table 1). The rate (percentage) of comorbid conditions was almost similar in all used DMARDs with no significant association between different DMARDs and presence of comorbid conditions (Table 4). Two previous studies from Saudi Arabia were reported concerning DMARDs use.16,17 Table 5 is a comparison between the current study and these 2 previous studies.
Association of different disease-modifying anti-rheumatic drugs (DMARDs) use (as single and/or combined) with comorbidity.
Comparison of disease-modifying anti-rheumatic drugs (DMARDs) use with other studies from Saudi Arabia.
Discussion
As stated in the methods and results, 6 DMARDs are involved in the current study; in this respect, 2 previous studies from Saudi Arabia were reported (Table 5). Al-Bishri et al16 studied 340 RA patients (Saudis and non-Saudis), with 86% receiving DMARDs. Safi et al,17 studied 200 Saudi RA patients (RF-ve), 97% were on DMARDs.
Two groups of DMARDs have been reported, including synthetic and biological (such as TNF-α blockers).1-4 In the current study, synthetic DMARDs were used including MTX, Hydroxychloroquine Sulphate (Plaquenil), Glucocorticoids (Prednisolone), Sulfasalazine, and Leflunomide (Arava), these synthetic DMARDs were combined with a biological anti-TNF agent (Adalimumab [Humira]). These DMARDs were also previously reported in Saudi Arabia,16,17 with additional biological DMARDs (MabThera [Rituximab] anti-CD20, and Infliximab [Remicade], which is anti-TNF),16,17 (Etanercept [Enbrel] anti-TNF)16 and an immunosuppressant (Azathioprine [Imuran)].17
The most commonly utilized drug was different between the 3 studies from Saudi Arabia. The most commonly used drug in the current study was MTX (71-76%), while Prednisolone (80.8%) was the highest in Al-Bishri et al’s study,16 and Plaquenil (46%) was the highest in Safi et al’s study.17 However, our results are in concordance with international reports considering MTX the most commonly used DARD worldwide.6,7 This high consumption of MTX (71-76%) is in concordance with Al-Bishri et al16 (74.4%), but not with Safi et al17 (25.5%). In this respect, it is advised that patients starting MTX therapy check their liver function tests18 as hepatitis was shown to be a contraindication for MTX therapy, and may deteriorate the condition of patients.19 Methotrexate is also teratogenic; thus, pregnancy should be avoided and monitored.8,10,18
Data in the current study showed approximate similarity with the previous studies16,17 regarding the rate of Plaquenil use. But, concerning Glucocorticoids and anti-TNF, our results were different from Al-Bishri et al16 who used more of both GC and TFN-blockers. In the current study, 7.2-12.5% of the patients received biological anti-TNF, compared with 49.2% by Al-Bishri et al.16 This difference can be attributed to the lower use of GC in the current study, keeping in mind that anti-TNF therapy in RA patients minimizes the risk of developing DM,20 which might occur as a side effect of GC therapy.21 Similarly, Safi et al17 reported low use of GC (9.5%) and anti-TNF (1.5%). Our results showed anti-TNF use in combination with different DMARDs (85%) or alone (15%); in this regard, it was advised that if DMARDs use was not effective after a trial of 3 months,4 they can be combined with biological agents, such as TNF-α blockers.1 Interestingly, anti-TNF (Humira) in the current study was used (as combination) only with MTX (19 times), as bi-therapy (60), as tri-therapy (30), or as tetra-therapy (10); this was in concordance with a previous report that a better effect can be obtained by a combination of MTX with anti-TNF than for MTX alone.14
Prednisolone was reported in 25-36% of the patients of the current study, compared with a high rate use by Al-Bishri et al16 (81), and a very low rate in the study of Safi et al17 (9.5). Glucocorticoids (such as prednisolone) are advised to be used in the short term, while waiting for slow-onset drugs to take effect;1 and as injections into individual joints.1 Although their long-term use reduces joint damage, different side effects have been suspected, such as osteoporosis,22,23 susceptibility to infections,1 hyperglycemia,21 HTN,24 and dyslipidemia.25,26
Using the odds ratio (OR) in the current study, we found no association between any DMARD use and presence of comorbidity. In contrast, Al-Bishri et al16 from Saudi Arabia, found a significant association between DMARDS use and comorbidity; as 60% of those with comorbid conditions (90% of HTN, dyslipidemia, DM, and osteoporosis) were on prednisolone; likewise 72% of those with hypothyroidism were on biological therapy. In combination with DMARDs, almost one third of the patients, of the current study used other drugs, folic acid being the most frequent. It is advisable to use low-doses of folic acid supplementation to reduce the side effects of MTX.27
Except prednisolone, none of the DMARDs showed a significant correlation with anti-MCV positivity indicating that usage of different DMARDs was not significantly distributed between positive and negative anti-MCV groups. Furthermore, anti-MCV positivity had no association with any of the different DMARDs use, indicating that anti-MCV positivity is not a useful predictor of DMARs use. However, the prognostic value of anti-MCV testing with respect to response to DMARDs can be a suggestion for a prospective longitudinal study in Saudi Arabia.
In conclusion, some of the findings of the current study are similar to the international results. First, MTX was the most commonly used DMARD; second, the addition of anti-TNF (a biological drug) for active disease, and folic acid to minimize the side effects. In this cohort, the pattern of use for all DMARDs was similar among Saudis and non-Saudis, which included 6 DMARDs, both synthetic and biological. The DMARDs use was either single or in combination; in both cases, MTX was predominant. The DMARDs combination was either with MTX or with Plaquenil (without MTX). There was a significant correlation between age and usage of Plaquenil. The DMARDs use did not depend either on anti-MCV positivity or on the presence of comorbid conditions, as there was no association between anti-MCV positivity, and DMARDs use, and no significant association between DMARDs use and presence of comorbid conditions. The current study provides valuable information and comparison with previous published DMARDs patterns in the western region of Saudi Arabia. In the current study, there are not enough data combining both DMARs use and disease activity; thus, a study of the association of DMARDs with disease activity is recommended. In addition, the prognostic value of the anti-MCV test with respect to response to DMARDs can be a suggestion for a prospective longitudinal study in Saudi Arabia.
Related Articles
Almoallim HM, Alharbi LA. Rheumatoid arthritis in Saudi Arabia. Saudi Med J 2014; 35: 1442-1454.
Atwa MA, Balata MG, Hussein AM, Abdelrahman NI, Elminshawy HH. Serum 25-hydroxyvitamin D concentration in patients with psoriasis and rheumatoid arthritis and its association with disease activity and serum tumor necrosis factor-alpha. Saudi Med J 2013; 34: 806-813.
Hanachi N, Charef N, Baghiani A, Khennouf S, Derradji Y, Boumerfeg S, et al. Comparison of xanthine oxidase levels in synovial fluid from patients with rheumatoid arthritis and other joint inflammations. Saudi Med J 2009; 30: 1422-1425.
Acknowledgment
We express our gratitude and appreciation to Dr. Suzan M. Attar (FRCPC) from the Department of Internal Medicine, King Abdulaziz Hospital, Jeddah, Saudi Arabia for her efforts and assistance with data collection and editing of the manuscript.
Footnotes
Disclosure. Authors have no conflict of interests, and the work was not supported or funded by any drug company.
- Received September 8, 2014.
- Accepted November 19, 2014.
- Copyright: © Saudi Medical Journal
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.