Abstract
Objectives: To investigate the effects of nicotine on orthodontic tooth movement and accompanying histological and immunohistochemical changes in rats.
Methods: An experimental study conducted at King Abdulaziz University, Jeddah, Saudi Arabia between 2013 and 2014. Thirty-two rats randomly divided into 4 groups. Three were experimental, received daily nicotine injections: group A: 0.37 mg/kg, group B: 0.57 mg/kg, and group C: 0.93 mg/kg. The control group (group D) received a daily injection of 0.5 mL saline. All rats were subjected to 30 g of orthodontic force on the maxillary left first molars and incisors using a nickel-titanium closed-coil spring. The distance between the 2 teeth was assessed before and after 14 days of force application. Histological, immunohistochemical, and histomorphometric assessments were performed on sections from groups C and D.
Results: Groups C (p<0.001) and D (p<0.001) showed the significantly greatest and least amounts of tooth movement . The results were statistically dose-dependent. Unbalanced resorption-apposition bone remodeling patterns and increased osteoclast cell distribution were observed in the nicotine group with significantly smaller percentages of bone surface areas mesially and distally (p<0.05). Immunohistochemical stains showed low alkaline phosphatase activity and intense tartrate-resistant acid phosphatase activity in the nicotine group.
Conclusions: Nicotine accelerated orthodontic tooth movement with unbalanced bone resorption and apposition patterns around the moving teeth.
Smoking and tobacco consumption is considered a major global health problem.1 Nicotine is one of many harmful substances in tobacco smoke affecting human health and among the 7000 poisonous chemicals discovered in tobacco.2 The effects of nicotine on bone remodeling were investigated in many studies using different methods of assessment.3-6 Nicotine was found to have a negative effect on the osseo-integration of implants7 and the healing and regeneration of bone defects.8-10 It was also reported to cause alveolar bone loss6 and periodontal tissue disease.11 Moreover, nicotine was documented to have undesirable effects during orthodontic treatment, which include compromised bracket adhesion,12 failure of miniscrews13 and negative effects on bone remodeling.14 Orthodontic appliances cause mechanical loading that can be transferred to the periodontal ligament, leading to inflammation, and generation of 2 different strains: compression and tension.15 Bone resorption is induced at the compression site, while bone deposition is induced at the tension site.15 Bone resorption and deposition are referred to bone remodeling,15 which is controlled by the activities of osteoclasts, osteoblasts, and osteocytes and regulated by biochemical and mechanical factors.16 Osteoblasts activation by mechanical loading is the first step in orthodontic treatment leading to the expression of mediators of osteoclasts formation and activation such as receptor activator of nuclear factor kappa B ligand (RANKL), which, binds to its receptor, RANK, on the surface of osteoclasts.16 The RANKL/RANK binding is essential for the osteoclasts and osteoclastogenesis.16-18 The effects of nicotine on bone remodeling during orthodontic tooth movement have not been widely investigated in the literature. Sodagar et al19 reported that nicotine accelerates orthodontic tooth movement in rats in a dose-dependent manner. In contrast, Shintcovsk et al14 found that nicotine decreases the number of osteoclast cells during orthodontic tooth movement, which contradict the results reported by Sodagar et al19 Thus, it can be stated that the reports available in the literature about the effects of nicotine on orthodontic tooth movement, especially at a cellular level, has produced contradictory results and needs further investigation.3,5,20-22 The purpose of the present study was to assess the effect of nicotine on orthodontic tooth movement using 4 measures: a) the amount of orthodontic tooth movement, b) histological changes in bone cells, c) bone cell distributions using immunohistochemical staining, and d) changes in the width of the periodontal ligament space and bone volume.
Methods
The study was approved by the Research Ethics Committee of the institute and conducted in accordance with EU Directive 2010/63/EU for animal experiments.
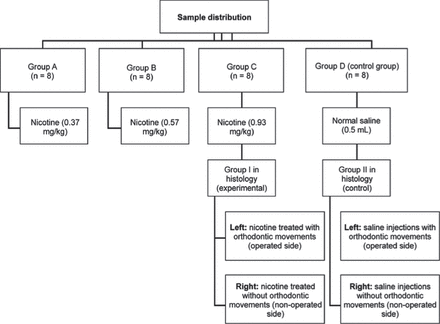
This is an experimental study conducted in 2013-2014 at King Fahad Research Center, Jeddah, Saudi Arabia. Based on the literature,19,23,24 and an 80% power for this experiment, a 32 12-week-old healthy male Wistar rats weighing 400 ± 20 g were found to be representative and thus used in the present study. They were randomly assigned to one of 4 groups (groups A, B, C, or D) according to the daily intraperitoneal injection regimen. The experimental groups received daily injections of nicotine as follows: group A, 0.37 mg/kg; group B, 0.57 mg/kg; and group C, 0.93 mg/kg. The control group (group D) received a daily injection of 0.5 mL of normal saline (Figure 1). The nicotine ditartrate salt used in the study (TRC, North York, ON, Canada) was dissolved in a normal saline solution and injected to the rats daily for 28 days. All rats were subjected to orthodontic tooth movement for 14 days. The injections started 14 days before force application and continued until the end of the clinical experiment. The effect of nicotine was assessed using 4 methods.
Sample distribution of groups A, B, C, or D according to the daily intraperitoneal injectionregimen an experimental study conducted at King Fahad Research Center, Jeddah, Saudi Arabia.
Clinical assessments of force application and tooth movement
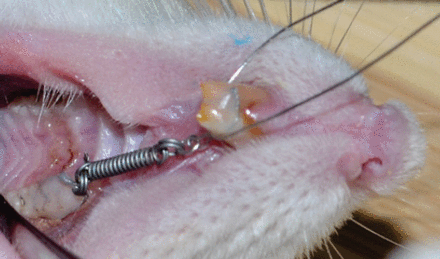
After an intraperitoneal injection of 40 mg/kg of sodium pentobarbital anesthesia (Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany), nickel-titanium (NiTi) alloy closed-coil springs (diameter: 0.25 inch; lumen: 0.9 mm; length: 4.0 mm; and load: 10 g) (Protect Medical Equipment Co., Ltd., Zhejiang, China) were placed between the maxillary left first molars and incisors following a procedure described in a previous study25 (Figure 2). Using an orthodontic force gauge (Forestadent, Pforzheim, Germany), the coils were activated to deliver 30 g of continuous force for 2 weeks. Tooth movement was assessed by measuring and comparing the distances between the left first molar and incisor before and after force application using an interproximal digital gauge (Mitutoyo Corp., Kawasaki, Japan).
Force application to the left first molar using a nickel-titanium closed-coil spring.
Histological assessments of bone cell distributions and periodontal ligament spaces
At the end of the clinical experiment, the rats in groups C and D were killed using an overdose of pentobarbital. The thorax and heart were exposed to permit perfusion fixation using 10% neutral buffer formalin via the aorta for 500 mL over 30 minutes. Then, the fixed head was decapitated and the maxilla was dissected and fixed for another 4 hours. The specimens were then demineralized in Ethylene diamine tetraacetic acid (EDTA) at a pH of 7.4 for 30 days and then rinsed with water. The tissues were dehydrated in a graded ethanol series, which was then replaced by xylene and infiltrated with paraffin wax. The mesiobuccal and distobuccal roots of the left (operated) and right (non-operated) first molar areas were chosen to investigate the histological changes. Accordingly, mesiodistal sections from the mesiobuccal and distobuccal roots at a 5-µm thickness were prepared and stained with hematoxylin and eosin (H&E) and used for detection of alkaline phosphatase (ALP) and tartrate-resistant acid phosphatase (TRAP) enzyme activity.
Immunohistochemical assessment of osteoblasts and osteoclasts
Alkaline phosphatase and TRAP enzymes were purchased from US Biological Life Sciences (United States Biological, Swampscott, MA, USA). Based on the protocol for their application, the sites of ALP activity are identified as brown-to-black and sites of TRAP activity are identified as black deposits of lead sulfide. Accordingly, ALP-positive osteoblasts and TRAP-positive osteoclasts were traced and assessed as intense, strong, moderate, or weak depending on a morphological descriptive scale.
Histomorphometric assessments of changes in the width of the periodontal ligament spaces and bone volume
Sections from the mesiobuccal roots on the operated (left) sides of groups C and D were chosen for histomorphometric assessment. The widths of the periodontal ligament spaces and the surface areas of the bone adjacent to the periodontal ligament were assessed on the mesial and distal surfaces of the mesiobuccal roots using the ImageJ software program (version 1.46; Rasband, W.S., National Institutes of Health, Bethesda, MD, USA). Photographs of the sections were taken using a digital camera (Olympus Optical, Osaka, Japan) to include the full length and width of the periodontal ligament spaces and the full surface area of the alveolar bone. To determine the periodontal ligament width, 3 measurements were taken from each photograph. Standardized lines were drawn to aid in locating and measuring the periodontal ligament spaces.
The total bone surface area was measured using 2 rectangles with standardized dimensions and locations drawn at the boundary between the periodontal ligament and alveolar bone using Image J. The sum of the surface areas of the 2 rectangles was considered to represent the total surface area of the tissues including the existing bone, newly formed bone (if present), bone marrow, and part of the periodontal ligament tissue. The surface areas occupied by marrow and periodontal ligament spaces were then subtracted from the total surface area to determine the surface area occupied only by bone.
Statistical analysis
Descriptive statistics were performed to report the results of all assessments. A one-way analysis of variance and Tukey’s honest significant difference test were calculated for mean comparisons of tooth movement between the 4 assessed groups. The correlation between nicotine dose and amount of tooth movement was assessed using Pearson’s correlation coefficient. The Mann-Whitney U test was used for mean comparisons of periodontal ligament widths, and the percentages of mesiobuccal root bone surface areas between the groups underwent histomorphometric assessment mesially and distally. The significance level was set at p<0.05. Data were analyzed using the Statistical Package for the Social Sciences software (SPSS v. 20.0; IBM Corp., Armonk, NY, USA).
Results
Effects of nicotine on the amount of tooth movement
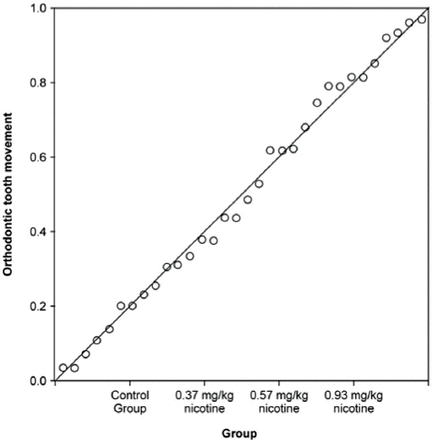
The one-way analysis of variance showed a statistically significant difference in the amounts of tooth movement among the 4 groups (p<0.001). Post hoc comparisons using Tukey’s honest significant difference test further indicated that the mean score in group C was the highest (mean: 0.82 ± 0.063 mm), while the control group (group D) showed the lowest mean score (mean: 0.23 ± 0.043 mm). However, the mean values in both groups A (mean: 0.50±0.057 mm) and B (M = 0.52±0.043 mm) were almost the same. Pearson’s correlation coefficient further revealed a significant correlation between nicotine dose and the amount of orthodontic tooth movement (R2 = 0.868; R = 0.93, p<0.001, predicted amount of orthodontic tooth movement in millimeters = 0.180 × nicotine dose + 0.247) (Figure 3).
The correlation between nicotine dose and amount of orthodontic tooth movement.
Histological findings
A) Descriptive histology of the non-operated side (maxillary right first molar)
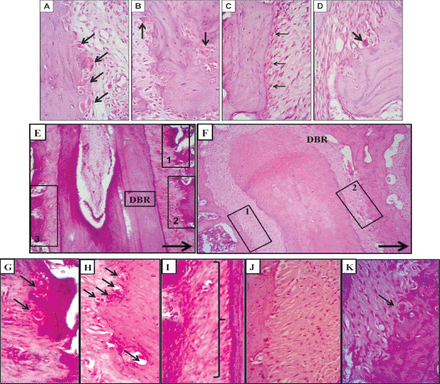
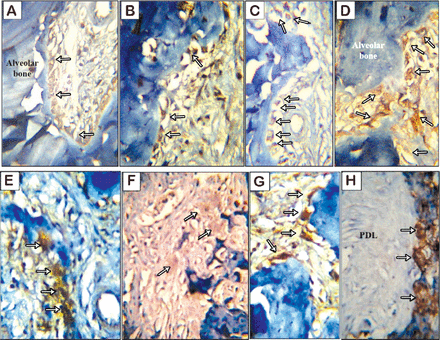
The experimental group showed more frequent occurrences of osteoclast cells. They were traced in random distributions without adhering to a single location on the alveolar bone. They were clearly identified by their large size, deep staining, and locations in resorption bays on the alveolar bone surface facing the periodontal ligament in association with the cancellous bone trabeculae of the interradicular septum. They were traced singly, or less frequently, in groups of 2 or 3 in one lacuna. On the other hand, the control group showed normal periodontal ligament structural components, a rich blood supply, and different cell populations of fibroblasts, osteoblasts, and occasional osteoclasts. The alveolar bone exhibited a typical structure with normal remodeling lines (Figures 4A - 4D).
Light microscopy showing A & B) osteoclast cells (arrows) in a resorption depression on the alveolar bone surface facing the periodontal ligament and on the bone interior of the non-operated experimental group. C & D) the detailed structures of the bones and cells of rat periodontium (non-operated control group). Note the density of the osteoblast cells (thin arrows) and occasional osteoclasts (thick arrows) (H&E staining, ×400). E-K) a mesiodistal section of the distobuccal roots of the maxillary left first molar (operated side). E) The experimental group demonstrates extensive resorption of alveolar bone on the mesial side of the root (insets 1 and 2) and bone apposition on the distal side (inset 3). F) The control group demonstrates resorption of alveolar bone on the mesial side of the root (inset 2) and bone apposition on the distal side of the root (inset 1). A thick arrow shows the direction of the applied mechanical movement (H&E, ×100). Light microscopy G and H) are higher magnifications of insets 1 and 2, respectively, and I) is a higher magnification of inset 3 from Figure E showing disorganized, inactive osteoblasts (right brace). J) A higher magnification of inset 1 from Figure F, revealing osteoblastic activity and bone apposition. K) A higher magnification of inset 2 from Figure F reveals an osteoclast cell (arrow) in a resorption depression on the alveolar bone surface facing the periodontal ligament (H&E, ×40). H&E - Hematoxylin and eosin
B) Descriptive histology of the operated side (maxillary left first molar)
The experimental group given the highest nicotine dose (0.93 mg/kg) showed decreased bone density around the mesiobuccal and distobuccal roots of the upper first left molar and a complicated remodeling pattern. That is, the bone trabeculae of the interradicular septum lacked continuous intercommunication and the bone marrow spaces appeared wider than those of the controls. The occurrence of osteoclast resorption bays was markedly increased. The periodontal ligament widths on the tension and apposition sides were greater than that on the compression side, reflecting an unbalanced apposition-resorption pattern. In addition, diminished osteoblast activity was observed on the tension side. Thus, the control group showed bone resorption with osteoclastic activity on the mesial sides of both the mesiobuccal and distobuccal roots of the upper first left molar, correlating well with the direction of movement. Bone apposition was clearly evident on both distal sides of these 2 roots. The resorption and apposition seemed balanced and the periodontal ligament width at the compression site appeared narrower than that of the tension site (Figures 4E - 4K).
Immunohistochemical findings
In the non-operated experimental group, ALP enzyme activity exhibited a moderate reaction and noticeable decrease rather than the strong activity observed in association with osteoblasts in the non-operated control group. The least ALP activity was associated with the operated experimental group, while the strongest activity was found in the operated control group (Figures 5A - 5D). Moreover, strong TRAP activity prevailed in the non-operated experimental group. The least activity of TRAP was found to be associated with the non-operated controls, while the strongest TRAP activity was found in the operated experimental group. However, the operated control group showed a strong-to-intermediate reaction of the TRAP enzyme (Figures 5E - 5H).
Light microscopy showing A) the non-operated experimental group shows moderate alkaline phosphatase (ALP) activity in osteoblasts on distal alveolar bone of the mesiobuccal root of the maxillary right first molar. B) The non-operated control group shows strong ALP activity in osteoblasts on the distal side of the same root. C) The operated experimental group shows weak ALP activity in osteoblasts on distal alveolar bone of the mesiobuccal root of the maxillary left first molar. D) The operated control group shows intense ALP activity in osteoblasts on the distal side of the same root (arrows) (ALP enzyme, ×400). E) the non-operated experimental group shows strong tartrate-resistant acid phosphatase (TRAP) activity in osteoclasts (arrows) adjacent to the inter-radicular bone between the mesiobuccal and distobuccal roots of the maxillary right first molar. F) The non-operated control group shows weak TRAP activity in osteoclasts on the mesial side of the same root. G) The operated experimental group shows intense TRAP activity of osteoclasts (arrows) adjacent to alveolar bone on the mesial side of the distobuccal root of the maxillary left first molar. H) The operated control group shows strong ALP activity in osteoclasts on the alveolar bone of the mesial side of the same root (TRAP, ×400).
Discussion
The current study assessed the effect of nicotine on orthodontic tooth movement and accompanying histological changes in a rat model. Although many differences between human and rat periodontal ligament and bone tissue have been documented, rats are still considered good models for orthodontic tooth movement.26 Yet the results obtained from such models should be considered with caution unless assessed in humans, or at least in higher animal model.
Nicotine doses tested in this study were selected according to Nociti et al,27 who found direct negative effects on the periodontal ligament at such doses, even without the presence of any irritating factor. The level and duration of force application used in the present study were determined based on those reported by Gonzales et al.25 They reported that tooth movement was significantly greater when 10, 25, and 50 g rather than 100 g of force was applied during a 14-day period of tooth movement.25 Thus, 30 g of force was used in our study for duration of 14 days. After determining the appropriate dose of nicotine that could create the highest difference (group C) from the control (group D), further assessment using histological and immunohistochemical testing was performed to compare group C with the control. Groups A and B showed insignificant difference between them, which indicates the small difference between the 2 doses.
Similar to the findings of many studies,20,21,24,28,29 our results showed increased osteoclast cell distribution and activity in the nicotine groups on both the non-operated and operated sides with a complex remodeling pattern. Such findings were explained by Henemyre et al28 by stating that nicotine stimulates osteoclast differentiation and resorption of calcium phosphate, which is the principal component of bone. Katono et al21 also reported that nicotine stimulates the resorption process that occurs during osteoid turnover by increasing the production of matrix metalloproteinases. Moreover, Tanaka et al29 found that the number of TRAP-positive multinucleated osteoclasts significantly increased with nicotine. On the contrary, Alder et al30 found that nicotine did not stimulate osteoclast cell formation in the bone marrow of rats.
Osteoblastic activity was diminished in the tension site of the experimental group when compared with the control group in the current study. These findings agree with the findings of Kim et al3 and others5 who reported that nicotine suppresses osteoblast proliferation. On the contrary, they disagree with the findings of Fang et al32 who found that nicotine enhanced the activity of ALP in osteoblastic-like cells and with those of Yuhara et al22 who reported that nicotine enhanced the rate of Ca+ deposition by osteogenic cells and ALP activity in a dose-dependent manner.
Alveolar bone density, as measured histomorphometrically was lower in the experimental group on both sides when compared with the control group in the current study. These findings agree with 2 other studies,23,32 which reported loss of alveolar bone around the molar furcation areas of rats, injected with nicotine.23,32 Such differences in bone density between the control and nicotine groups might explain the faster mechanical tooth movement observed in the experimental groups. In the current study, the histomorphometric findings further supported and confirmed the histological findings. The increase in periodontal ligament width on the distal (tension) side and decrease in bone surface areas on both the mesial and distal sides indicated greater bone resorption than the equivalent expected bone apposition. Hapidin et al24 similarly reported a significant decrease in trabecular bone volume, trabecular thickness, mineralizing surface, mineral appositional rate, and bone formation after injecting rats with a high dose of nicotine (4 mg/kg) over a long duration (4 months), while osteoclast surfaces and eroded surfaces increased.24 They also reported that serum interleukin-1 and interleukin-6 levels, which are bone-resorbing factors, increased significantly in the nicotine group.24 Their experiment was not conducted under orthodontic tooth movement conditions.
As mentioned earlier, few studies have previously assessed the effect of nicotine on orthodontic tooth movement in rats.14,19 The current study agrees with the results of Sodagar et al19 who found that nicotine accelerated orthodontic tooth movement in a dose-dependent manner. However, Sodagar et al19 study assessed tooth movement without any histological evidence. In addition, their conclusion on the acceleration of nicotine to orthodontic tooth movement in dose-dependent manner was not confirmed statistically. In our study, this correlation was found highly significant (p<0.001). In contrast, our study disagrees with the study of Shintcovsk et al14 who recently investigated the effects of nicotine on orthodontic tooth movement histologically in rats. They found that nicotine decreased the numbers of osteoclast cells during orthodontic movement in rats injected with 2 mg/kg nicotine for 3, 7, 14, and 21 days. These contradicting results could be attributed to the extremely higher nicotine dose used in the study by Shintcovsk et al.14 In addition, TRAP staining was used in our study, which is a more accurate method of assessing osteoclastic activity as TRAP is a lysosomal enzyme that is secreted by osteoclasts to induce osteoclastogenesis.33
Study limitation
The use of rat model, which makes it difficult to generalize the obtained conclusion on humans. Yet, our results highlighted multiple complications that smokers might experience during and after orthodontic tooth movement.
Human clinical trials and higher animal experiments using more advanced techniques for assessment of nicotine effect on orthodontic tooth movement are needed to provide an in-depth understanding of managing orthodontic patients who are smokers. Undesirable nicotine levels, orthodontic force levels, the rate of orthodontic tooth movement, as well as the effects of nicotine on root resorption under such conditions also require further investigation.
In conclusions, nicotine was found to accelerate orthodontic tooth movement resulting from unbalanced bone resorption and apposition around moving teeth in rats. Studies in higher animals and human clinical trials are needed to confirm these findings. These findings can be considered the nucleus for developing evidence-based protocols and guidelines for managing orthodontic patients who are smokers.
Footnotes
Disclosure. Authors have no conflict of interests, and the work was not supported or funded by any drug company. This study was funded by the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, Saudi Arabia (Grant # 254/345).
- Received April 21, 2016.
- Accepted July 20, 2016.
- Copyright: © Saudi Medical Journal
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.