Abstract
As of January 2016, 1,633 laboratory-confirmed cases of Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infection and 587 MERS-related deaths have been reported by the World Health Organization globally. Middle East Respiratory Syndrome Coronavirus may occur sporadically in communities or may be transmitted within families or hospitals. The number of confirmed MERS-CoV cases among healthcare workers has been increasing. Middle East Respiratory Syndrome Coronavirus may also spread through aerosols generated during various dental treatments, resulting in transmission between patients and dentists. As MERS-CoV cases have also been reported among children, pediatric dentists are at risk of MERS-CoV infection. This review discusses MERS-CoV infection in children and healthcare workers, especially pediatric dentists, and considerations pertaining to pediatric dentistry. Although no cases of MERS-CoV transmission between a patient and a dentist have yet been reported, the risk of MERS-CoV transmission from an infected patient may be high due to the unique work environment of dentists (aerosol generation).
In 2014, Memish et al1 reported the clinical presentation and outcome of Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infection in 11 Saudi pediatric patients. However, MERS-CoV was initially isolated from a 60-year-old Saudi patient in September 2012.2 Middle East Respiratory Syndrome Coronavirus is a novel beta coronavirus of the Coronaviridae family that causes a severe respiratory disease with a high fatality rate.3-6 As of January 2016, 1633 laboratory-confirmed cases of MERS-CoV infection and 587 related deaths have been reported by the World Health Organization (WHO) globally.7 The male-to-female ratio of the affected patients was 2:1, with a median age of 49 years.8 The incubation period for human-to-human transmission ranges from 2-15 days.9
The symptoms of MERS-CoV infection range from being asymptomatic to severe pneumonia, acute respiratory distress syndrome, septic shock, and multi-organ failure, leading to death. The symptomatic patients may have fever, cough, chills, throat soreness, myalgia, arthralgia, vomiting, or diarrhea.10 Furthermore, men over 45 years of age, patients with comorbidities, and healthcare workers have been reported to be high-risk groups for MERS-CoV infection.11 The virus has been detected in respiratory, gastrointestinal and other bodily secretions,12,13 as well as in air samples, which indicates the possibility for airborne transmission.14
Several studies have reported MERS-CoV infections among healthcare workers4,6,15,16 and children.1,17 Viral infections, such as severe acute respiratory syndrome (SARS-CoV), may be transmitted to healthcare workers from infected patients through aerosols.18 Considering that several types of dental equipment, such as handpieces, air-water syringes and ultrasonic scalers, produce considerable amounts of aerosols, the potential for the spread of infections from patients to dentists or dental assistants is high.19 This review is an attempt to discuss MERS-CoV infection among children and those providing dental treatment to them, including precautions and considerations pertaining to the practice of pediatric dentistry.
MERS-CoV infection in healthcare workers
The 3 patterns of MERS-CoV transmission suggested in the literature include the following: a) sporadic cases occurring in communities; b) transmission within families; and c) nosocomial transmission.20 Healthcare-related MERS-CoV transmission is associated with high morbidity, extended use of mechanical ventilation and fatality rates of up to 65%; this type of transmission can be attributed to shortcomings in observing stringent infection control protocols.4,6,15,21 Furthermore, MERS-CoV has been reported to be viable in hospital-like environments for up to 48 hours with a stability that is unaltered during aerosolization.22 Among 200 healthcare workers’ contacts identified in Al-Hassa, Saudi Arabia, MERS-CoV infection was confirmed in 2 cases.4 A Saudi study20 compared healthcare worker and family contact with laboratory-confirmed MERS-CoV patients and reported a lower rate (1.12%) of infection among the healthcare workers than among the families (3.4%).
Healthcare workers could be infected with MERS-CoV through exposure in the community or at their workplace,1,6,20 they could be diagnosed late,1,6 and they could remain asymptomatic or mildly symptomatic.6,8,16 Furthermore, unsuspected cases entering healthcare facilities have been considered the main source of MERS-CoV.4 Considering these aspects and that healthcare workers may continue to work regardless of being symptomatic,15 the possibility of transmitting the infection to their patients is also high.
MERS-CoV infection in children
Memish et al1 reported that the age of 11 Saudi pediatric patients who presented clinically with symptoms of MERS-CoV infection ranged from 2-16 years with a female-to-male ratio of 2.7 to 1. Of the 11 cases, 2 were symptomatic, and 9 were asymptomatic with no underlying comorbidities. Another report by Thabet et al17 concluded that although MERS-CoV infection presents with a wide range of clinical manifestations, the mortality rate in children is lower than that in adults. A review on the effects of Coronavirus infection in children concluded that Human Coronavirus infections may be associated with respiratory symptoms and may also involve central nervous system. The authors suggested that the clinical and genetic traits of Human Coronavirus infection among children should be monitored closely for early prevention.23
Dental considerations. Bioaerosols in dental practice
Bioaerosols are defined as a suspension of biological particles in gaseous media.24 Apart from radiation exposures, dermatitis, eye injuries, and musculoskeletal and respiratory disorders, other occupational health risks exist in dentistry. These risks include percutaneous exposure incidents and exposure to infectious diseases, such as those that may be present in bioaerosols.25 Subgingival scaling of periodontally involved teeth with ultrasonic scalers may produce aerosols containing blood.26 One study reported that ultrasonic scalers and tips produced significantly more aerosol and splatter than a handheld curette, regardless of the scaler type used.27 Miller et al28 studied the characteristics of blood-containing aerosols generated by common powered dental instruments. The author concluded that all the recovered particles could contain the hepatitis B virus and be inhaled and retained (20-100%) in the human respiratory system.
The repeated and chronic exposure to bioaerosols generated during certain dental procedures and the relatively small particle size contribute to an increased risk of infection among dental professionals.29 Furthermore, the protection provided by surgical masks worn by dental professionals may be low for small particles; in addition, these masks may not fit perfectly in clinical practice.30 A study reported that 15-83% of plasma aerosol particles ranging from 0.06-2.5 microns in size passed through the filters of 9 makes of surgical masks used by dentists.28 These factors may play a role in the airborne route of viral infections among dentists and clinical dental auxiliaries.
The likelihood of detecting, reporting, documenting and publishing dentistry-associated infections is relatively less.31 Although no definitive evidence of an extensive public health hazard from exposure to dental unit waterlines has been reported.32 Few case reports have suggested a definitive link between exposure to contaminated dental unit waterlines and Legionella infection.33,34
Infection control in dental practice
A review by Scully and Samaranayake35 on the emerging and fluctuating viral diseases in the new millennium concluded that infection control plays an equally important role in the practice of dentistry as do the understanding of oral manifestations and the diagnosis and management of viral infections. While differences exist in the virology, epidemiology, and clinical outcomes of MERS-CoV and SARS-CoV infections,36 the clinical symptoms of MERS-CoV resemble those of SARS-CoV, apart from acute renal failure.37 Although these results cannot be directly interpreted for a definitive conclusion, the management protocols for different MERS-CoV infection-associated with clinical scenarios for dental professionals may be similar to those of SARS-CoV.
The implications of SARS-CoV for general dental practitioners, the significance of droplets and aerosols in disease transmission, the recommended management protocols for SARS-CoV infection and specific infection control measures have been well described by Li et al.38 A comprehensive review on universal and standard precautions has been published elsewhere39 and is beyond the scope of this article, infection control measures pertaining only to pediatric dental practice in the context of MERS-CoV infection are discussed here.
In pediatric dental practice, effective infection control measures for the prevention or minimization of viral infection transmission can be implemented by a) controlling the gag or cough reflex; b) reducing aerosol/splatter generation; c) managing contaminated air and; d) improving personal protection. The gag or cough reflex may be stimulated by certain procedures, such as posterior intraoral and bite-wing radiographs and taking impressions. Orthopantomographs or oblique lateral views may be considered instead of intraoral radiographs for screening,40 whereas oral mucosa in very sensitive patients may be anesthetized before taking impressions.41 Sedation may also be considered to control gag reflex.42
Varying levels of physical, intellectual, emotional and social development of children and adolescents are major challenges for dentists especially attending to their psychological needs within adequate infection control regimen. Toys provided for pediatric patients may be a potential source of cross-infection. Soft toys are more likely to be contaminated, difficult to disinfect and may re-contaminate quickly compared to hard-surfaced toys. Furthermore, restraining devices used to control movements of pediatric patients such as Velcro fasteners may also be contaminated and should be disinfected accordingly.43 Extra-oral evacuation devices and special aerosol reduction devices may be used in combination with ultrasonic scalers to reduce the amount of aerosol produced.44,45 In high-risk cases, chemomechanical caries removal46 or the atraumatic restorative technique47,48 may be utilized to prevent or reduce aerosol and splatter generation. In addition, high-volume evacuation removes infectious droplets at the source as they are emitted; thereby, minimizing, or preventing their dispersion in the air. To maintain their efficacy, the filters in the suction apparatus should be cleaned every day, and the exhaust air should be vented outside to prevent the recirculation of contaminated air.38
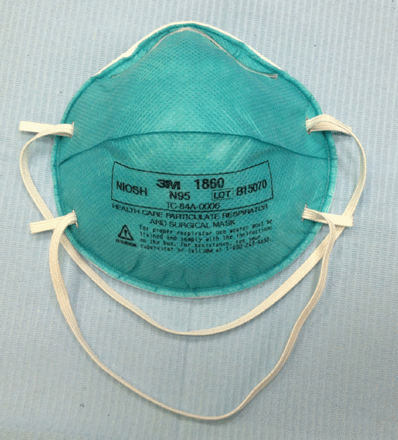
Contaminated air can be managed by improving dental clinic ventilation and/or by disinfecting the air. An ideal airflow pattern combined with a minimum of 3 air changes per hour has been recommended for dental clinics.49-51 Moreover, although its use in dental clinics is unconfirmed, ultraviolet germicidal irradiation may be installed and is effective against fungi, viruses, and bacteria, namely, tubercle bacilli and anthrax.38,49 On the other hand, measures of improving personal protection include washing hands frequently before and after treatment, using disposable barriers, dispensing instruments and materials just before treatment (thereby preventing particles from settling on the surfaces), and sterilizing soiled instruments.38 After each patient visit, surfaces may be disinfected using hospital-grade disinfectants, which are effective against coronavirus.52 Personal protective equipment, such as gowns, hair covers, masks, gloves, shielded face masks and shoe covers, should be used as appropriate.51 A higher level of respiratory protection should be considered, especially with aerosol-generating procedures. The use of N95 respirators offers a certain level of protection against the airborne transmission of SARS-CoV, although the exact level of protection offered by these respirators for individuals may vary.53 Examples of personal protective equipment such as face mask with plastic shield (Figure 1) and N95 respirator (Figure 2). These respirators may also provide some level of protection against MERS-CoV and may be utilized instead of surgical masks in dental clinics. Apart from these measures, vaccination of pediatric dentists and staffs working in pediatric dental clinics against measles, mumps, varicella and rubella may be necessary for their protection from these viral infections.54
Face mask with plastic shield.
The N95 respirator.
In conclusions, considering the unique work environment of dentists, which involves close patient contact and aerosol production, the risk of MERS-CoV transmission from an infected patient is high. Children are also prone to MERS-CoV infection. As the number of MERS-CoV cases may increase in future, pediatric dentists should be well informed and educated about not only the signs and symptoms of the condition but also how to follow stringent infection control measures in these cases.
Acknowledgment
The author would like to extend his sincere gratitude to Prof. Amjad Wyne, Department of Pediatric Dentistry and Orthodontics, College of Dentistry, King Saud University, Riyadh, Saudi Arabia for critically evaluating this review.
Footnotes
Disclosure. Authors have no conflict of interest, and the work was not supported or funded by any drug company.
- Copyright: © Saudi Medical Journal
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.