Abstract
This review summarizes the development of head and neck cancer resection and reconstruction. The developments in the treatment of cancer patients are reflected in their surgical outcomes, in addition to functional and aesthetic improvements. New technologies, such as surgical simulation and planning, minimally invasive surgery, and microsurgery have been added to the field to improve surgical resection of the tumor and reconstruction. The field is still growing to optimize the management of head and neck cancer.
Head and neck cancer represent the sixth most frequent malignancies. Worldwide more than 500,000 new cases are diagnosed annually, along with 300,000 deaths. Head and neck cancer predominantly affects men, with a male: female ratio of up to 10:1. There is also an increased risk of developing this type of cancer.1,2 Tobacco smoking, alcohol consumption and human papillomavirus (HPV) infection are the principal risk factors for the development of head and neck cancers.3-5 In the United States, more than 60% of oropharyngeal cancers are associated with HPV infection. Head and neck cancers that are associated with HPV infection usually affect nonsmoking white male patients who are younger than patients with unrelated HPV cancers and who have a higher socioeconomic status and a history of multiple sexual partners.3,6 Most head and neck cancers (more than 90%) are squamous cell carcinomas originating from the epithelium, which are the most common cancer in males and the third most common cancer in females.6,7 This type of cancer may be found on the lips, oral cavity, pharynx, larynx, sinuses, nasal cavity and the salivary glands.8 More than two-thirds of patients present with regional lymph node involvement.9 Early diagnosis of this cancer leads to higher survival rates; the 5-year survival rate drops from 83% to 37% when the diagnosis is made late. Squamous cell carcinoma associated with HPV presents with higher survival rates at 3- and 5-year intervals when compared with squamous cell carcinoma associated with smoking.1
Surgery, chemotherapy, and radiotherapy are the common treatment modalities for head and neck cancer. The purpose of this review was to summarizes the development of surgical resection of head and neck cancer with the reconstruction of the resected human part.
Review
Oral cancer was seldom reported in medical reports before the introduction of tobacco in the 16th century.10 In 1650, Hayes Martin wrote the first report detailing oral cancer treatment.11 Then, in 1664, Marchetti described the first tongue resection procedure as a treatment for cancer.12 Then, in 1712, Joseph de la Charriere recommended cauterization as a treatment for cancer patients.13 After that, the first cancer hospital was established in France in 1740.14 In 1790, the awareness of the importance of lymph node metastasis appeared in the literature.14 In 1860, the dynamics of lymphatic spread were studied.15 In 1846, successful removal of cervical cancer was performed by Dr. Warren, and this was the beginning of major surgical procedures.12 Advances in surgical procedures continued in the 20th century. These advances in surgery and medicine occurred during the first half of the century due to the 2 world wars.12
Surgical resection is the primary treatment modality for oral cancer. Tumor resection involves removal of the lesion with a wide margin of normal tissue; involved cervical lymph nodes also need to be removed.16-18
Head and neck cancer resection
The radical neck dissection for head and neck cancer was first descried in 1906 by Dr. George Crile from the Cleveland Clinic in Ohio.19 His procedure involved the removal of all the lymphatic structures between the mandible and the clavicle. After that, Dr. Hayes reviewed the procedure of Dr. Crill by performing 1450 neck dissections in 1951 at the Memorial Hospital in New York City. His method started with a midline bisection of the lower lip, then by segmental mandibulectomy and in-continuity radical neck dissection.20
Forty percent of head and neck cancer patients present with cervical lymph node metastasis. Cervical lymphadenectomy is a procedure that involves removal of groups of lymph nodes from the neck to decrease disease burden.21,22 Neck dissection can be therapeutic or elective, depending on the presence of clinical or radiographic lymphadenopathy. Elective neck dissection is aimed at ruling out the possibility of cancer metastasis, which may eliminate the need for adjuvant therapy after surgery. Shah23 stratified cervical lymph nodes into 7 group levels; submental (I), submandibular and upper jugular (IIa, IIb), middle jugular (III), lower jugular (IV), posterior triangle (Va, Vb), central compartment (VI), and superior mediastinal (VII).21,23-25
Currently, the classical radical neck dissection is seldom performed for the head and neck cancer patients, where all the lymph nodes in the anterior and posterior triangles, the submandibular salivary gland, and the tail of parotid salivary gland, the spinal accessory nerve, the internal jugular vein and the sternocleidomastoid muscles will be removed. It also involved the removal of the spinal accessory nerve, the internal jugular vein and the sternocleidomastoid muscles. However, the modified radical neck dissection where involved the removal of the cervical lymph nodes in levels I through V without the removal of 3 aforementioned anatomical structures. Currently, cervical lymph nodes removal with the preservation of non-lymphatic structures is more commonly performed through selective neck dissection (levels I-IV) or supraomohyoid neck dissection (levels I-III).21
Conventional head and neck surgery involving neck dissection is performed via a U-shaped or Y-shaped incision through the neck, leavening a visible scar that may have a psychological impact on the patients.26,27 In 2012, Kim et al28 introduced robotic-assisted surgery for lateral neck dissection for squamous cell carcinoma patients that resulted in a good aesthetic appearance and avoided the long scar that was associated with the conventional open technique without compromising the surgical completeness and oncological outcomes.
Surgical treatment modalities for head and neck cancer require complete tumor excision along with excision of the surrounding margins and are usually performed via a transcervical approach and may require a lip-split mandibulotomy or mandibulectomy.29,30
Classical open cancer resection or minimally invasive resection can be performed depending on the specific cancer anatomy and characteristics. Classical open surgery is less favorable in young patients due to the aesthetic impairments caused by the visible scars that this procedure may produce.29,31
Minimally invasive surgeries
In the late 20th century, minimally invasive surgeries were developed. Trans-oral endoscopic head and neck surgery is a minimally invasive surgery for the oropharynx through a trans-oral route that is performed either with conventional instruments or by laser resection.17,32 Trans-oral laser microscopy and trans-oral robotic surgery are the specific approaches used. These can be performed without external skin incisions, so there is no need to gain access through a mandibulotomy or transmandibular approach, which significantly reduces the postoperative morbidity. The surgical time may be shorter than that of the transcervical approach. These techniques provide a highly magnified view of the tumor, which permits confident resection of the tumor.33,34
Trans-oral laser microsurgery (TLM)
The first laser microlaryngoscopy procedure was described in 1971.35 In 2001, Steiner described this approach for more widespread use.36 Surgical resection is performed through direct laryngoscopy using a carbon dioxide (CO2) laser. The laser beam is transferred through the endoscope and is absorbed by water at the tissue-laser interface, which then coverts it into thermal energy and allows precision tissue cutting. This type of resection is performed under a binocular microscope that allows close visualization of the tumor, coupled with flexible microsurgical instruments that enable a better approach to the resected area. This technique requires the surgeons’ understanding of the complete anatomy and extent of the tumor and the surrounding structures.32
Trans-oral robotic surgery (TORS)
In 2009, the United States Food and Drug Administration approved the use of the da Vinci surgical system.37,38 This involves real surgical procedures performed in a virtual environment, where the surgeon sits at a remote console and can manipulate an endoscope and 2 additional instruments that are placed in the patient’s mouth. The surgeon can control every movement of robot’s instruments. The robot’s arms carry interchangeable working instruments, such as grasping forceps and an electrocautery tool. The patient’s mouth is kept open with the help of a suitable oral retractor, and an endoscope or camera is introduced through the mouth. The flexibility of the robotic arms allows suturing of structures in low visibility areas, which is not possible with the standard techniques. Countertraction and suction performed by a bedside assistant are needed throughout the surgery.30
Patients with retrognathia, class II dental occlusion, limited cervical extension and prominent maxillary dentition have limited oropharyngeal access, thus considered not suitable for trans-oral robotic surgery. So, tumors arising from the tonsillar fossa, the lateral pharyngeal wall, the glossopharyngeal sulcus, and the lateral tongue base can be resected with the assistance of trans-oral robotic surgery due to their suitable position for this technique.30
Most surgical resections of head and neck lesions can compromise the patient’s appearance as well as certain functions; thus, reconstruction of the defect area is necessary. Jaw reconstruction restores the continuity of the jaw, separates the oral and nasal cavities, and restores a stable base for the oral cavity and the structures to which muscles are attached, leading to improved mastication, speech, swallowing, tongue function, breathing and definition of the lower third of the face, which affects aesthetics.39-41 In addition, surgical reconstructions of the jaws produces proper phonation and deglutition functions compared with reconstruction with prosthetics.42,43 In 2010, Moreno et al43 compared microvascular free flap reconstruction and palatal obturator reconstruction for maxillectomy defects and concluded that better functional outcomes were associated with free flap reconstruction. Techniques for the reconstructing the defect area vary from the simplest primary closure to the more complex free vascularized flaps.44
History of head and neck reconstruction
Bardenheuer first reported a non-vascularized cortical bone graft in 1892 that used the mandible itself to rebuild its defect. It was used in World War I, harvesting bone from the rib and the tibia. In particular, cancellous bone in a metallic tray or block graft was used.45
Before the 1950s, resected cancer was left without reconstruction. Reconstruction was performed only when no early local recurrence developed.45 In the late 1950s, the first free flap procedure, which involved the removal of tissue from the donor site and transplantation in another site of the body with anastomosis of the vessels, was performed.46,47
Advances in head and neck reconstruction emerged in the 1960s with the introduction of the myocutaneous pedicle flap, and afterward, the description of the pectoralis major myocutaneous flap was described in 1979.48 The transferring and reconstruction of the defects with the flap provided the patient with healthy, vascularized tissue and recovered the resected areas. In the 1990s, free flap reconstruction was the dominant technique for cancer defect reconstruction.45
The traditional method of jaw reconstruction is challenging, particularly with regard to bone graft shaping. Malposition of the bone graft can negatively affect the facial symmetry, appearance, support, occlusal function, masticatory movement and dental rehabilitation. Manual remolding of the graft can prolong the period of ischemia.49
Digital technologies and surgical simulation provide 3-dimensional (3D) virtual models of the resected and grafted areas based on preoperative high-resolution computed tomography (CT) data with specialized printers using rapid prototype modeling technology.49 Three-dimensional printing was first described by Hideo Kodama in 1981 when he started manufacturing 3D plastic models.50 In 1990, Mankovich et al51 described the rapid prototyping technique for medical use, transferring the engineering method to the surgical field. Surgical planning was improved, which facilitated resection, flap harvesting, and graft positioning.
Through surgical planning and simulation, a 3D printed surgical template can be fabricated to guide the procedure of tumor resection, graft harvesting, and shaping and positioning of the graft in the defect area.52,53 It depends on the adaptation of certain anatomical landmarks, and then osteotomies are performed through the metal cutting slots on the surgical template.54
Surgical simulation and planning lead to exact planning and good communication between the resection and reconstruction team, which results in increased accuracy, improved bone to bone contact, decreased operating room time, lower surgeon stress, reduced ischemia time in the microvascular free flap reconstruction and enhanced treatment outcomes.55-67
After the resection of head and neck cancer, more than 50% of the resections require reconstructions of the defect and involve bony and/or soft tissue reconstruction.7 Maxillomandibular resection may involves only an edge (marginal) or complete segment (segmental) reconstruction. A marginal mandibulectomy can be reconstructed with local flaps or skin graft, whereas a segmental mandibulectomy requires an osteocutaneous flap.16,68
Soft tissue reconstruction
Soft tissue reconstructions are usually based on the defect size after the resection of cancer. Primary closure healing or healing by second intention for small defects and skin grafts for larger defects are the simplest options for reconstruction. For larger defects, full or partial thickness skin grafts can be utilized, using different flap methods depending on the defect site and size.16
Several soft tissue flaps can be harvested from several sites of the patient’s body, like the radial forearm, lateral arm, ulnar forearm, anterolateral or lateral thigh, latissimus, dorsi, jejunum, omentum, rectus abdominis, scapula, and temporal parietal fascia.47,69,70 Some axial regional pedicle flap may be preferred because microvascular anastomosis is not necessary and shorter harvesting times are needed for this technique compared with the free flap procedure, such as latissimus dorsi and pectoralis major myocutaneous flaps; however, but the bulkiness and inflexibility of these flaps makes them inferior to the vascularized radial free forearm flap.71
Myocutaneous trapezius flap
The myocutaneous trapezius flap is a thin and large pedicle flap that has been used for intraoral defects and posterior occipital defects. It has a wide arc of rotation, which make it an option for head and neck reconstructions.72,73 In 1980, Baek et al74 reported the use of the trapezius flap for a facial defect pedicle flap reconstruction. It is an alternative reconstructive flap that is suitable for patients who cannot undergo microvascular free flap reconstruction. It can also be used for primary or salvage reconstruction surgery.75
Vascularized radial free forearm flap
In 1983, Soutar et al76 reported the use of the radial free forearm flap for intraoral reconstructions to replace the oral mucosa. This fasciocutaneous flap is now a popular option for soft tissue reconstructions. Because it is usually a mobile, thin, and pliable large paddle of skin without added bulkiness, it can be used for a variety of external skin and intraoral defects, such as defects of the palate, the floor of the mouth and the tongue. The long pedicle flap (up to 20 centimeters) with large caliber vessels facilitates surgery without repositioning of the patient during surgery, which decreases the surgical time.77-79 Full thickness defects of the cheeks or lips can be reconstructed using a folded fasciocutaneous radial forearm free flap.80
Hard tissue reconstruction
Osteocutaneous flaps consist of soft and hard tissue, and bone grafts can be harvested from different donor sites of a patient’s body, such as the fibula, iliac crest, radius and scapula.81-87
Scapular flap
In 1986, Swartz et al88 first reported the use of an osteocutaneous scapular flap for mandibular and maxillary reconstructions. The bone is harvested from the lateral border of the scapula with scapular and parascapular skin paddles. The flap is harvested in the lateral decubitus position and remains vascularized by its pedicle until reconstruction is performed to prevent flap ischemia.89 A 2 team approach is difficult because of the need for intraoperative patient positioning.90 While a limited amount bone of low quality can be harvested and multiple osteotomies cannot be tolerated, this technique does provide large amount of soft tissue. Therefore, this method is an option for mandibular reconstructions in cases were a fibular flap is unsuitable or in cases that require extensive soft tissue reconstruction.91,92
Vascularized fibular free flap
The vascularized fibular free flap is the first option and is the gold standard for reconstructions of the mandible or maxilla due to the shape and quality of the bone, which is suitable for contouring and allows dental rehabilitation through the placement of dental implants.83,93-98 It was described first by Hidalgo in 1989 for mandibular reconstruction, and its successful depends on the surgeon’s skills, decisions and trial and error.82 The surgical plane for resection and reconstruction is dependent on a diagnosis made with 2D images with clinical measurements that may affect the position and the shape of the graft.99,100
The fibular graft has multiple advantages including the following: a large amount of bone and soft tissue are available for harvesting (approximately 20-26 cm); the bone tolerates multiple osteotomies; the graft can be easily shaped; there is a long and anatomically reliable vessel pedicle with a wide vessel diameter; the technique is associated with low donor site morbidity; and the location of the donor site facilitates a 2 team approach without the need to reposition the patient during the surgery.101-103 The site and the length of the graft depend on the defect size.100 The bicortical structure seems to increase the long-term success of dental implants placed in the graft, along with good primary stability of the implants.93
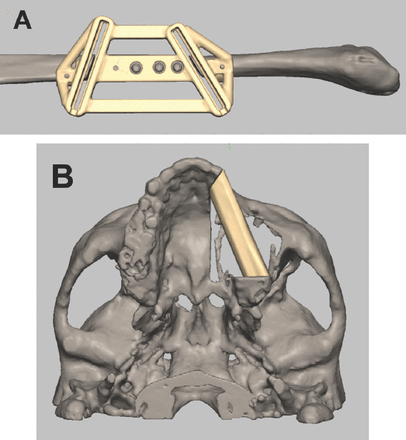
The traditional surgical procedure of transferring the straight fibula into the curved mandible often resulted in imprecise contouring, condylar positioning, and symmetry of the lower third of the face.41,101,104 With advanced surgical technologies, the fibula can be redesigned and reshaped with the help of 3D printed surgical templates, along with the use of osseointegrated dental implants that are placed at the time of the reconstruction (Figures 1A & 1B).63,105
Surgical planning and simulation using 3-dimentional printed surgical template for A) fibular cutting guide and B) reconstruction of partial left maxillectomy.
Wang et al41 studied a comparison of virtual planning surgery and conventional surgery for mandibular reconstruction with a vascularized fibular free flap and found that virtual surgical planning produced a more accurate mandibular reconstruction than conventional surgery.
The limitation of the fibula graft is the limited height of the graft (rarely more than 1.5 cm), which is about half of the native mandible, which makes it insufficient to replace the skeletal base and alveolar ridge. Therefore, the iliac crest flap, due to its height and shape, emerged as a good option for bony reconstruction.106
Vascularized iliac crest flap
The iliac crest is commonly used as a source of non-vascularized bone graft.45 In 1979, Taylor first described the use of the vascularized iliac crest flap for mandibular reconstruction. Then, it became widely favored by surgeons due to the large amount of bone volume, rich cancellous blood supply and compact cortex.107-109 Through surgical simulation and planning, the ideal bone graft site inside the iliac crest that is the most similar to the missing bone with regard to the outline and size can be identified thus avoiding the risk of wasting a bone graft.81,110 It is an optimal choice to reconstruct defects related to the inferior orbital rim, the vertical process of the maxilla and the zygomatic arch.111 However, it is not an alternative to the fibular and scapular free flaps due to the difficulty in harvesting the graft and the thin and immobile skin paddle.45
Osteocutaneous radial forearm free flap
In 1979, reconstruction with an osteocutaneous radical forearm free flap was introduced for head and neck reconstruction. This technique is suitable for short bony defects such as defects of the maxilla or the mandible.79 On the other hand, it is not recommended for large head and neck reconstructions due to the limited bone stock and the morbidity of radius harvesting, including the possibility of donor radial fracture.112,113
Pre-bent reconstruction plates
Since the twentieth century, the surgical plates have been used for bone segments stabilization during head and neck reconstruction surgery. Conventionally, surgical plates are available in standard formats and are manually bent by the surgeons during surgery to match the individual bone anatomy.114
The manual plate-bending procedure can be a time intensive and energy consuming technique, particularly for inexperienced surgeons.114 On occasion, surgical plates need to be bent repeatedly, particularly in complicated cases, which may concentrate internal stress within the plate that leads to fatigue under masticatory loading, resulting in complications, such as plate fracture, corrosion, screw loosening and bone resorption.115 To overcome the complications of conventional surgical plates, 3D technologies have facilitated the production of patient-specific pre-bent surgical plates.63,116-119 Pre-bent selective laser 41 sintering (SLS) or milled titanium surgical plates with specific configurations, including the 3D architecture, width, thickness and screw holes, can be individualized for each patient using the patient’s original printed contours.120 This type of plate has threaded screw holes with different angles and can tolerate a greater number of screws than a conventional plate and has a minimum distance between screws of 5.5 mm compared with the 8 mm of a conventional plate.121 In addition, this type of plate can greatly facilitate graft positioning into the resected area, avoiding dental malocclusion due to the accurate contouring of the surgical plate.100,122 Moreover, damage to important structures, such as tooth roots or nerves, can be avoided by determining the locations of each fixation hole.123
Personalized titanium meshes can be used to restore the orbital floor and facial contour during surgical reconstruction after a maxillectomy with orbital floor involvement to avoid displacement of the eyeball and diplopia due to changes in the orbital volume. The titanium mesh is highly biocompatible and can be contoured into the resected areas.49,124,125
Dental rehabilitation
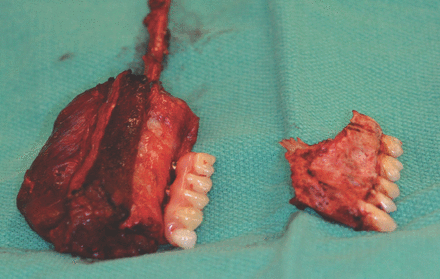
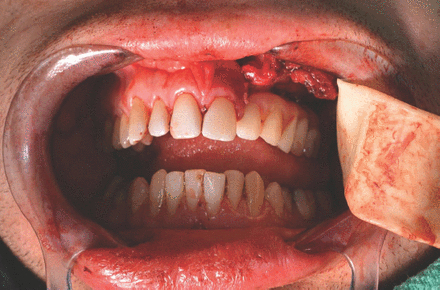
Planning for dental implants and the type of dental prosthesis that is needed to replace missing teeth after a resection is a part of the virtual surgical planning and simulation for maxillary or mandibular resection and the reconstruction procedure. The implants can be placed at the time of jaw reconstruction, which will reduce the time for dental rehabilitation, enhance optimum bone exposure during implant placement and eliminate the need for hyperbaric oxygen after radiotherapy.126 Placing the dental implant within the vascularized bone graft before reconstruction facilitates the correct positioning of the graft within the defects by putting the patient into occlusion using a provisional prosthesis or fitting template for the implants (Figures 2 & 3).53,93
Provisional prosthesis supported by dental implant on fibular graft after partial left maxillectomy.
Provisional prosthesis facilitates correct positioning of the graft.
Free flap perfusion assessment and monitoring
The success of the reconstructions depends on the quality of perfusion of the harvested flap and the rapid identification and salvage of a failing flap.127,128 Doppler ultrasonography and angiography have improved surgeons’ abilities to make decisions regarding microvascular reconstructions.129 Laser-assisted fluorescent angiography is now used to monitor perfusion intraoperatively. A perfusion map can be constructed by administering an intravenous injection of indocyanine green (ICG) to the patient, which fluoresces on exposure to a laser light emitted by the machine and is detected by a high-speed imaging system that is sensitive to the ICG wavelength.130 The immediate assessment of vascular anastomosis and venous and arterial flow allows the identification of thrombi and an assessment of the quality of perfusion, which are determinants of early flap necrosis.130 The assessment of tissue oxygenation by near-infrared spectroscopy is one of the advancements in postoperative flap assessment that is a sensitive, specific and non-invasive method.131 Measuring the hemoglobin saturation at the capillary level by visible white light spectroscopy is also an advancement in monitoring. Smartphones can also be used for flap monitoring through infrared thermography cameras.132,133
In the 21st century, new advances in reconstruction of head and neck defects are developing. In 2004, Lendeckel et al134 reconstructed a calvarial defect through the use of autologous stem cells (adipose) combined with the patient’s own cancellous bone and fibrin glue. The resorption of non-vascularized bone grafts or donor site morbidity of vascularized bone flaps during maxillofacial reconstruction can be avoided through the use of stem cells to produce the bone.135 The harvesting of adipose-derived stem cells is easy, where it can be performed by liposuction or lipectomy. The cells can differentiate into different cell lineages, such as adipogenic, osteogenic, chondrogenic, neurogenic, myogenic, cardiomyogenic, angiogenic, tenogenic and periodontogenic cell types.136 After a period of implantation of these cells in a titanium mesh that is seeded with bone marrow stem cells in certain areas of the patient’s muscles, vascularized bone pieces of the size and outline needed to perform reconstruction can be produced. The resultant bone can tolerate dental implants with excellent outcomes.137,138
In conclusion, through the past decades, surgical resection and reconstruction for head and neck cancer have developed significantly. Surgical simulation, 3D printing, microvascular surgery and minimally invasive surgery have improved the surgical outcomes and have led to superior functional and aesthetic outcomes. Undoubtedly, the future will bring more developments in the field to optimize the treatment of head and neck cancer.
Acknowledgment
The author acknowledge the Institute for Reconstructive Sciences in Medicine (iRSM), Alberta, Canada for providing the photos. Also the author acknowledges the American Journal Experts company for the professional editing of the manuscript.
Footnotes
Disclosure. Authors have no conflict of interests, and the work was not supported or funded by any drug company.
- Copyright: © Saudi Medical Journal
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.