Abstract
Objectives: To investigate the period prevalence and risk factors for clinically important prescription and monitoring errors among adults managed in community care in Saudi Arabia (SA).
Methods: This retrospective cohort study used electronic health record (HER) data. A random sample comprising of 2,000 adults (≥18 years old) visiting Family Medicine clinics in King Faisal Specialist Hospital and Research Center (KFSH & RC), Riyadh, SA, was selected. Data collection took 3 months (October December 2017). Descriptive analyses and logistic regression modeling were performed using STATA (version 14) statistical software.
Results: The overall period prevalence of medication errors over 15 months was 8.1% (95% confidence interval [CI] 6.5-9.7). Risk factors that significantly predicted overall risk of patients experiencing one or more medication errors were: age ≥65 years, male gender, Saudi nationality, and polypharmacy (defined as the concurrent use of ≥5 drugs).
Conclusions: Clinically important medication errors were commonly observed in relation to both drug prescription and monitoring.
Patient safety is a public concern in healthcare systems across the world.1 Medication errors are a major problem across care settings, including home, ambulatory, and primary care (henceforth community) settings.1 The World Health Organization (WHO) has identified medication errors as key focus areas for the enhancement of patient safety in community settings.2 A recent systematic review revealed considerable variations in the prevalence rates of medication errors in community settings. This result, at least in part, reflects variations in: i) the definitions of medication errors used in studies, ii) the populations studied, iii) the methodologies employed for error detection, and iv) the outcome measures studied.3 This systematic review also highlighted the absence of studies focusing on medication errors in community settings in the Kingdom of Saudi Arabia (KSA). The pharmacist-led information technology intervention for medication errors (PINCER) trial is among the world’s first randomized studies that aimed to reduce the risk of medication errors in general practice. A validated tool for the measurement of medication errors was developed by Avery et al4 and was used in the PINCER trial in the United Kingdom (UK). This trial shows that the PINCER intervention is more effective than simple feedback for reduction of the numbers of patients at risk from prescribing and monitoring errors in general practice.
The objectives of this study were to investigate the epidemiology of clinically important errors in medicine management, as defined by the PINCER trial4 and risk factors for clinically important errors among adults managed in community care in SA.
Methods
The current study was divided into 3 phases: a feasibility phase, pilot retrospective cohort phase, and retrospective cohort study. The feasibility phase involved the identification of sites in SA with ambulatory electronic health record (EHR) data for the investigation of issues pertaining to the accessibility and completeness of data, and which provided the opportunity for the dataset to be used in outcome evaluation (Table 1). The PINCER trial focused on a pre-specified list of clinically important errors in prescription and monitoring stages of medicine management.4
The pilot phase involved testing: i) sample generation, ii) data extraction, and iii) outcome assessment on a randomly selected sample of 200 patients. This article focuses on the pilot phase and the main retrospective cohort study.
The research protocol, data collection sheet, and waiver of informed consent (in place of individual informed consent) were approved by the Clinical Research Committee and the Research Ethics Committee (REC) of the Office of Research Affairs, King Faisal Specialist Hospital and Research Center (KFSH & RC), Riyadh (project # 2171 060), KSA.
Several ambulatory care centers in Riyadh were contacted for fieldwork selection. Family Medicine clinics in KFSH & RC, Riyadh, SA were selected.
A random sample of patients visiting the Family Medicine clinics in KFSH & RC was generated, and the follow-up was performed retrospectively over the 15 months before data extraction. Data collection took 3 months (October 2017 to December 2017). Electronic records were selected using a random number table that was generated using the “simple random sample without replacement” function in STATA (version 14).
The inclusion criteria were: i) Saudi and non-Saudi adults aged 18 years or older, ii) patients who had been registered with the Family Medicine clinics at KFSH & RC for at least 15 months prior to data extraction, and iii) patients recorded as receiving at least one prescribed or over-the-counter (OTC) medication. These medications were checked against the Saudi Food and Drug Authority (FDA) list of human medications and were subsequently classified into prescription or OTC medications.5 Patient records were excluded if they did not fulfill the inclusion criteria.
The patients’ recorded baseline characteristics were as follows: i) age, ii) gender, iii) nationality (Saudi, non-Saudi), iv) diagnosis or underlying conditions, v) OTC medication use recorded at any point during the 15 months, and vi) polypharmacy (≥5 medications at any point during the 15 months). The exposures of interest were the risk factors, and prescription and/or OTC drug.
The outcome variables were: i) period prevalence of the primary, secondary, composite secondary, and revised updated outcome measures, ii) patient and medication-related risk factors (age, gender, nationality, polypharmacy and OTC medicine use), and iii) physician-related risk factors: age (18-50 years, ≥51 years), gender, nationality (Saudi, non-Saudi), number of physicians involved in a patient’s care (one, more than one), certification (American, British, Canadian, Jordanian, or none), and number of years of experience (1-9 years or ≥10 years). For details on the primary, secondary, composite secondary, and revised updated outcome measures are summarize in Table 1.4,6
We then compared the results of the cohort study with the baseline results of the UK PINCER trial,4 which were derived through QResearch database interrogation.7
Data sources/measurement
After the selection of a random sample from the Family Medicine clinics, in-depth EHR screening, involving the assessment of diagnostic, medication list, and laboratory data, was conducted.
Development of a data collection tool and process
A paper-based data collection form was used to extract summary descriptions of all the relevant information available in the EHRs to gather each patient’s demographics and outcome measures (Appendix 1). The information obtained was transferred to an Excel spreadsheet for analysis. The electronic data sheet was stored in a password-protected computer and no patient-identifying information was recorded.
Data collection form of medication errors study.
Manual data extraction from the EHRs, involving 200 records, was independently undertaken for the pilot retrospective study. For the main cohort study, data extraction was performed for all 2,000 records, while a second trained reviewer undertook the independent assessment of a random 10% of the sample of records.8,9 Any discrepancy or disagreement was discussed and resolved through double-checking of records or arbitration if a decision could not be reached.
To reduce the risk of selection bias in sampling, simple random sampling was employed. The independent evaluation of a sample of records was designed to minimize the risk of information bias.10
For the cohort study, the largest sample size that was feasible given: i) the time available, ii) resources, iii) research team number, and iv) the manual method employed for data extraction resulted in a total of 2,000 records. A sample size of 10% or more of the major study size is commonly deemed adequate for pilot studies;11 thus, 200 patient records were randomly selected for the pilot phase.
Data access and data cleaning methods
Data access and cleaning methods were used as per the PINCER trial protocol.6,7 The electronic data sheet was checked for errors in data entry, outliers, and missing data. An inventory of medical record numbers and each patient’s code number was used to ensure that the same patient was not included in the dataset more than once.
Statistical analysis
Microsoft Excel was used to manage and process data; STATA (version 14) was used to analyze the data.
The overall period prevalence rate of patients with at least one error was calculated as: “the number of patients experiencing one or more medication error at any time during the 15-month period (numerator)/the total number of patients in the study population (denominator)”.12
The overall period prevalence rate of medication errors was calculated as follows: “the number of medication errors at any time during the 15-month period (numerator)/the total number of patients in the study population (denominator)”.
The prevalence of each outcome measure was described using: i) numerators, ii) denominators, and iii) percentages at patient level, as detailed in the PINCER trial protocol.6,7 To illustrate patients’ demographic characteristics and diagnoses, descriptive statistics in terms of frequency counts and proportions were used. To evaluate the association between the risk factors and outcomes, we performed logistic regression modeling. The results of the regression analysis were presented in terms of odds ratios (OR) with their 95% confidence intervals (CIs). For logistic regression modeling, the dependent variable was defined as the presence/absence of the outcome or, more fully, the presence/absence of patients at risk of clinically important errors. To determine the agreement between the 2 independent data extractors, a Kappa coefficient was calculated. A Kappa score: is a measure of inter-rater agreement for categorical variables.13 Landis and Koch suggested that a Kappa value lower than 0.40 denotes poor-to-fair agreement, 0.41-0.60 moderate agreement, 0.61-0.80 substantial agreement, and 0.81-1.00 almost-perfect agreement.14
This study follows the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) checklist15 and the REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) Statement (Appendix 2) for reporting the findings.16
The RECORD statement-checklist of items, extended from the STROBE statement that should be reported in observational studies using routinely collected health data.
Results
Family Medicine clinics in KFSH & RC were selected following the feasibility assessment. Five hundred records meeting the inclusion criteria were reviewed. All the necessary information from each patient’s record was available in the Integrated Clinical Information System. In the feasibility study, all the outcomes were observed at least once in a total of 500 patients, except for outcomes 7, 8, and 10 (Table 1). However, none of the outcome measures were excluded from the pilot and main studies, because it was considered possible that outcomes 7, 8, and 10 may appear when screening a higher number of records.
The findings from this phase of the research indicated that the pilot study was feasible and likely to provide a random sample, and all the information needed for the outcomes was available in one system. Continuation to the pilot and main phases of the study was therefore initiated without the exclusion of any of the outcome measures.
Pilot retrospective study
In the pilot retrospective study, a random sample of 200 records was selected from the Family Medicine clinics in KFSH & RC. The overall period prevalence rate of patients with at least one medication error over 15 months was 10% (95% CI 5.8-14.2). The overall period prevalence rate of medication errors over 15 months was 16% (95% CI 8.2-23.8). The pilot study suggested that clinically important errors commonly occurred in the medicine management of adults. The highest risk of prescription errors was observed in asthma patients who had been prescribed a ß-blocker. A monitoring error was found in one patient receiving lithium for at least 3 months; the patient did not have a recorded check of their lithium concentrations in the previous 3 months. Risk factors that significantly predicted the overall proportion of patients at the risk of medication errors were age ≥65 years and OTC medication use; however, the obtained data suggested that other factors may be identified in the larger planned follow-up study.
The main retrospective cohort study
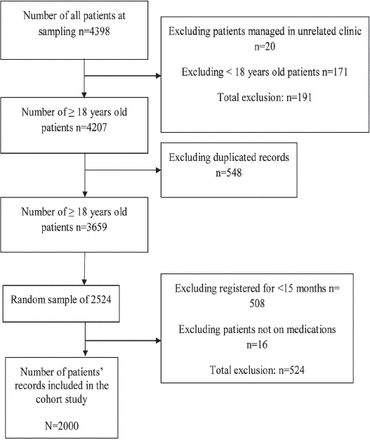
A total of 4,398 patients visited the Family Medicine department one month prior to data collection. The required information from 2,000 electronic records was collected after the exclusion of patients who do not meet the inclusion criteria (Figure 1).
Cohort study flowchart outlines and sample enrollment.
The percentage of adults in the age range 18-64 years was 83.85% and the percentage of those aged 65 years or older was 16.15%. The majority of the study population was of Saudi nationality (67.2%). Table 2 summarizes the participants’ characteristics. The agreement between the 2 independent data extractors dealing with the 200 EHRs was substantial (Kappa 0.8). All discrepancies were resolved by discussion and the double-checking of records.
Cohort study participants’ demographic characteristics.
Table 3 summarizes the prevalence of each outcome measure. A total of 162 prescribing/monitoring errors were found during the study period. The overall period prevalence of patients with at least one medication error over 15 months was 5.85% (95% CI 4.8-6.9), while the overall period prevalence of medication errors over 15 months was 8.1% (95% CI 6.5-9.7).
Cohort study period prevalence of each primary, secondary, composite and revised updated outcome measure described using numerators, denominators, and percentage, at patient level.
Risk factors that significantly predicted the overall proportion of patients at risk of experiencing medications errors were: i) age of ≥65 years, ii) male gender, iii) Saudi nationality, and iv) using ≥5 drugs (Table 4). Risk factors that significantly predicted the overall proportion of patients who were at risk of experiencing medication errors were physicians’ male gender and Saudi nationality (Table 5). Table 5 also summarizes the risk factors for individual errors.
Cohort study association between patient and medication-related risk factors and patients at risk of errors outcomes. (Data obtained from logistic regression model).
Cohort study association between physician-related risk factors and patients at risk of errors outcomes. (Data obtained from logistic regression model).
Discussion
Clinically important errors were observed commonly in medicine management. A random sample of 2,000 patient records was selected, resulting in the identification of 162 clinically important errors in medicine management. The overall period prevalence of patients with at least one medication error over 15 months was 5.85% (95% CI 4.8-6.9), while the overall period prevalence of medication errors was 8.1% (95% CI 6.5-9.7). The highest risk of prescription errors was in Outcome 2a: ‘patients with asthma who had been prescribed a ß-blocker’. However, for monitoring errors, the highest risk was in Outcome 7: ‘patients receiving lithium for at least 3 months, who did not have a recorded check of their lithium concentrations in the previous 3 months’.
Patient and medication-related risk factors that significantly predicted the risk of errors were as follows: i) age ≥65 years, ii) male gender, iii) Saudi nationality, and iv) using ≥5 drugs. Physician-related risk factors that significantly predicted the risk of errors included male gender and Saudi nationality.
Strength
First, the list of clinically important errors in the prescription and monitoring stages that were used in this study was validated and then developed according to a systematic review, with inputs from research studies and experts, and a consensus on the overall burden and severity of iatrogenic harm in primary care, in the PINCER trial.17-19 Second, data collection of the total pilot study sample and 10% of the sample size of the cohort study was independently undertaken by 2 reviewers, resulting in substantial agreement. Third, patient, medication, and physician-related factors that contribute to the risk of medication error occurrence were considered. Fourth, Outcome 5, methotrexate, was observed more frequently in the cohort study than the pilot study. Fifth, the large, nationally representative cohort of adults was followed-up over a 15-month period. Finally, this is the first epidemiological cohort study to focus on a pre-specified list of clinically important errors in community care settings in SA.
Study limitations
This study has some limitations that should be considered. First, outcomes 8 and 10 were not observed; this could be attributed to the low prescription rate of amiodarone to cardiac arrhythmia patients. Second, data collection was performed manually due to the inability to ensure the accuracy and quality of patients’ information gathered electronically. Manual data extraction was also employed to avoid delays associated with the generation of the required anonymized data electronically from the electronic medical records department in KFSH & RC. Third, the results may not be generalizable because the study was conducted in a single community care setting in KSA. Fourth, the actual rates of use of these medications may be unknown as a large number of OTC medications can be brought in from outside the hospital and may not be recorded by physicians. Fifth, there is a risk of information bias, as the investigators relied on only EHR information for the identification and assessment of the outcomes of interest. Finally, there is inconsistency in the precision type between the period prevalence measure in the present cohort (namely, 95% CI) and that in the PINCER trial (namely, IQR); as a result, we compared the proportions alone without precisions.
Interpretation in the light of the wider published literature
Our results were compared to the baseline characteristics of the PINCER trial, as derived from the QRESEARCH database, which is one of the largest aggregate general practice electronic databases worldwide, comprising 487 general practices.20 The overall period prevalence of the first 12 clinically important errors in medicine management was 3.4% (95% CI 2.2-4.6) in this research, compared to the 0.9% in the PINCER trial.20
The distribution of each estimate for the outcome measures is as follows: In this study, higher period prevalence estimates were observed for the following: Outcomes 2a and 2b: asthma and ß-blocker, Outcome 6: warfarin and international normalized ratio, Outcome 7: lithium and lithium level, Outcome 11: at least one prescription error, and Outcome 12: at least one monitoring error. In this study, we could not estimate the rates for the following outcomes, because there were no events: Outcome 1: peptic ulcer and NSAID without an ulcer-healing drug, Outcome 3: ACE inhibitor / diuretics and laboratory test, Outcome 4: venous or arterial thromboembolism and arterial thrombosis and combined oral contraceptives, and Outcomes 5a and 5b: methotrexate and full blood count, and methotrexate and liver function test. For Outcome 8, amiodarone and thyroid function test, we observed no patients on amiodarone. This may reflect both the differences in the healthcare services provided in KSA and the UK and the varied methods of the extraction of data and outcomes between the 2 studies. In the baseline characteristics of the PINCER trial, data were collected prospectively through a computer-recorded method and the level of accuracy and completeness was shown to be high.20,21 However, in this study, the data were collected retrospectively through manual data extraction. Akbarov et al22 in a cross-sectional study using linked records in the UK general practices, used 22 medication safety indicators (18 prescribing indicators with an overall prevalence as 5.45% and 4 monitoring indicators with an overall prevalence as 7.65%). In order to compare our study results with the findings from the previous study,22 it is important to have a consistent definition of numerator and denominator. Only 13 consistent indicators can be compared with the outcome measures employed in this study. The other 9 indicators were not used in our study, so a comparison between the overall outcome measures’ estimate and other study22 overall outcome measures’ estimates were not established. This present study found higher period prevalence estimates for the following: Outcome 2a (asthma and ß-blocker), Outcome 6 (warfarin and INR), Outcome 13 (aged ≥65 years using NSAID without an ulcer-healing drug), Outcome 15 (warfarin/NOAC and NSAID), and Outcome 19 (heart failure and NSAID). In this study, we could not estimate the rates for the following outcomes, because there were no events: Outcome 1 (peptic ulcer and NSAID without ulcer-healing drug), Outcome 3 (ACE inhibitor/diuretics and lab test), Outcome 4 (venous or arterial thromboembolism and arterial thrombosis and combined oral contraceptives), Outcomes 5a and 5b (methotrexate and full blood count and methotrexate and liver function test), and Outcome 21 (eGFR <45 and NSAID). For outcome 8 (amiodarone and thyroid function test), no patient in this study was on amiodarone. For (outcome 18: long-acting beta-2 agonist inhaler [excluding combination products with inhaled corticosteroid] to a patient with asthma who is not also prescribed an inhaled corticosteroid), all the study patients were on combination products with the inhaled corticosteroid.
Implications for research, policy, and practice
For healthcare professionals, there is a need for the following: i) training, education, and monitoring with the involvement of medication safety pharmacists in the community, ii) the implementation of computerized prescription with software integration for the detection of clinically important errors in medicine management during prescription entry, iii) the provision of a record of current medication lists for each patient in the community, and vi) empowerment and education among patients and the public, particularly among those with chronic diseases and polypharmacy, to increase the knowledge of medication safety. For patients, tools and technology should be employed, particularly for monitoring and follow-up, as most medication errors occur in this stage due to irregular outpatient visits; there is a need for patients’ current medication lists to be shown at each pharmacy visit. Further study is required for the following: i) to replicate the outcome measures in different community care contexts in KSA, to increase the generalizability of our findings and ii) to further explore error-related adverse events, and their causes and prevalence, in community care settings in SA. Consideration should also be given to undertaking interventional studies aimed at reducing the risk of medication errors. A trial based on the PINCER trial could be conducted in the KFSH & RC. Such an initiative would have to be modified to a parallel group design, as opposed to the cluster, randomized control design used in the PINCER trial. A random sample of records of individuals could be selected and randomized to receive either simple feedback or pharmacist intervention. The first challenge would be to identify, by manual or computer-generated methods, those patients who are potentially at risk of clinically important errors in medicine management. In the simple feedback arm of this trial, physicians would be given manual or computer-generated feedback on patients who are at potential risk of clinically important errors, together with brief written educational materials explaining the importance of each type of error. In the pharmacist-intervention arm, pharmacists should provide simple feedback plus educational outreach “academic detailing”, while considering the human error theory and provide support in order to correct and prevent medication errors. The choice of manual or computer-generated methods is likely to be challenging, as the computer-generated method has not been used till date in the KFSH & RC. This proposed research initiative should aim to do the following: i) calculate the prevalence of the outcome measures before and after the intervention and ii) decrease the number of clinically important errors as much as possible.
In conclusions, this study shows that clinically important medication errors occur commonly, and such mistakes could potentially harm patients’ health. Patient-related risk factors that significantly predicted the overall proportion of patients at risk of experiencing medication errors were as follows: i) age ≥65 years, ii) male gender, iii) Saudi nationality, and iv) using ≥5 drugs. Physician-related risk factors that significantly predicted the overall proportion of patients at risk of errors were male gender and Saudi nationality. Future research should aim to replicate these findings in other community care contexts in SA, to further explore any associated error-related adverse events, and also to develop and evaluate an intervention aimed at decreasing the incidence of clinically important errors in medicine management in SA.
Acknowledgment
We are grateful to Sarah Rodgers, from the PINCER trial team, for her help in answering our clinical inquiries regarding the outcome measures; to Sarah Al-Hathlool for her help; to Dr. David Boorer for proofreading. We would like to thank the Deanship of Scientific Research and Researchers Support and Services Unit at King Saud University, Riyadh, Saudi Arabia for the technical support and English language editing. We would like to thank the Prince Abdullah bin Khalid Celiac Disease Research Chair, Vice Deanship of Scientific Research Chair, King Saud University, Riyadh, Saudi Arabia.
Footnotes
Disclosure. Authors have no conflict of interests, and the work was not supported or funded by any drug company. The project was financially supported by the Prince Abdullah bin Khalid Celiac Disease Research Chair, Vice Deanship of Scientific Research, King Saud University, Riyadh, Kingdom of Saudi Arabia.
- Received October 16, 2018.
- Accepted January 1, 2019.
- Copyright: © Saudi Medical Journal
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.