Abstract
One of the most significant problems facing maternal and children health worldwide is preterm birth (PTB). Although strategies to increase the survival of premature infants have significantly improved in the past few decades, they have yet to be successful. Nine years ago, the use of progesterone in pregnancy was approved by the United States Food and Drug Administration (FDA) for PTB prevention. This paper reviews the recent evidence supporting the use of progesterone in pregnancy for PTB prevention and provides guidelines for its use in daily clinical practice. The guidelines address multiple current controversial areas regarding the prevention of PTB to aid physicians with their clinical decision-making practice, including the use in multifetal gestation, different formulations, safety in pregnancy, dose and route of administration.
The definition of preterm birth (PTB) is delivery before 37 weeks’ gestation. Since PTB is a major cause of worldwide neonatal mortality and morbidity, its prevention is of high priority in obstetric care.1 It is estimated that 15 million preterm babies are born annually, with PTB rates ranging from 5% to 18%.2 In Saudi Arabia, the PTB rate was approximately 6% in 2010.3 Nationally, PTB complications are also the foremost reason behind neonatal mortality, accounting for approximately 54% of the cases in 2008.4
Progesterone’s role in PTB prevention has been the subject of several randomized controlled trials (RCTs) over the past few years. These trials examined pregnancies at high risk for spontaneous PTB (SPTB) in the setting of a prior SPTB or presence of short cervix, as confirmed by the use of ultrasound during the routine mid-trimester scan.
The purpose of this guideline is to provide evidence-based information for practitioners involved in the care of pregnant women with regard to the use of progesterone to reduce recurrent SPTB risk and aid in clinical decision-making.
Evidence
Relevant RCTs, systematic reviews, and meta-analysis were searched for in MEDLINE, PubMed, and Cochrane Library, with a restriction to articles published between 1996 and 2019. Relevant Medical Subject Headings (MeSH) combined with a Keyword search were used to comb the databases. The search was limited to the English language.
Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach was used to assess the quality of evidence and strength of recommendations, which is now considered the standard in guideline development (Table 1).
Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach was used to assess the quality of evidence and strength of recommendations.
Mechanism of action
Progesterone displays biological effects on the myometrium and uterine cervix.5 Through animal studies, several researchers have proposed that progesterone withdrawal or a decline in its action is a key control mechanism for cervical ripening.6-9
Though the precise mechanism of action of progesterone in the prevention of PTB is still unidentified, there are 2 widely accepted mechanisms. The first is a local anti-inflammatory effect at the maternal-fetal interface that counteracts the inflammation process leading to PTB, while the second is the ability to modulate other biological processes implicated in cervical ripening.6,10
Safety data on progesterone use in pregnancy
The safety of progesterone use in its synthetic or natural form in the first trimester of pregnancy is outside the scope of this guideline, and thus, will not be discussed.
Current evidence suggests that starting progesterone therapy after 14 weeks’ gestation is safe for both the mother and fetus. The OPPTIMUM trial found that at the age of 24 months’ children exposed to vaginal progesterone in utero have no significant differences in neurodevelopmental outcomes compared to controls in the same age group.11 Similarly, the observation of children with in utero exposure to 17-alpha-hydroxy-progesterone caproate (17-OHPC) up to 4 years of age did not reveal any detrimental effects.12
What is the role of progesterone in singleton pregnancies with no history of PTB and unknown cervical length?
A small group of 168 women with no history of PTB and unknown cervical length (CL) was given a weekly dose of 17-OHPC beginning at 16-20 weeks. A beneficial effect compared to placebo was not observed.13
We could not find an RCT that examined the effect of vaginal progesterone in this group of women.
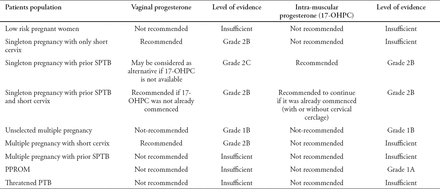
In low risk women with singleton gestations, no history of SPTB and unknown or normal CL there is no evidence to support the use of progesterone for PTB prevention. Therefore, it is not recommended to use progesterone in low-risk women for prevention of PTB. (Grade 2C)
What is the role of progesterone in singleton pregnancies with no history of PTB, but a short cervical length at 18-24 weeks?
Cervical shortening detected with transvaginal ultrasound in the mid-trimester is a strong predictor of SPTB.14
Grobman found that a weekly 250 mg intramuscular injection (IM) of 17-OHPC in nulliparous women with singleton gestations and transvaginal ultrasound cervical length (TVU CL) of <30 mm at 16-22 weeks was not superior to placebo for PTB prevention at <35 weeks and <37 weeks.15
Several large RCTs have shown a significant reduction in SPTB risks among asymptomatic women who received vaginal progesterone following transvaginal ultrasound diagnosis of a short cervix.16-19
A meta-analysis by Romero et al20 showed a significant PTB risk reduction in women with a TVU CL equal to or less than 25 mm who started vaginal progesterone (from 27.5% to 18.1%).20 Romero et al21 included the OPPTIMUM trial in their recently published meta-analysis and found a decrease in the risk of PTB at less than 33 weeks in singleton gestations with a sonographic short cervix of less than 25 mm (14% versus [vs.] 22%). Furthermore, vaginal progesterone significantly decreased PTB risk at <36 weeks of gestation. Vaginal progesterone was also found to decrease the risk of neonatal respiratory distress syndrome, birthweights of less than 1500 g and 2500 g, and neonatal intensive care unit admission.21
Romero et al21 also found that a vaginal progesterone gel with a daily dose of 90 mg has a similar compared to a daily 200 mg of vaginal progesterone tablets in the reduction of PTB risk and composite neonatal adverse outcome.21 Therefore, there is not enough evidence to support the use of one vaginal progesterone preparation or dose over the other.
Women with singleton gestations, no history of SPTB, and sonographic short cervix (TVUCL <20 mm), and <24 weeks of gestation may benefit from vaginal progesterone to reduce PTB risk, perinatal morbidity, and mortality (Grade 2B).
Patients can be given 90 mg vaginal progesterone gel or 200 mg vaginal progesterone tablet or suppository once per day, preferably at bedtime. (Grade 2B).
Using weekly intramuscular 17-OHPC injection is not recommended for this group of women (Grade 2C).
What is the role of progesterone in singleton pregnancies with prior PTB?
In women with a history of SPTB, most studies suggest a significant risk reduction in PTB, perinatal mortality, and major morbidity among pregnant women treated with progesterone regardless of its route of administration.22-32
Meis et al30 found that an IM weekly administration of 250 mg of 17-OHPC, starting at 16-20 6/7 weeks of gestation, was linked to a 34% decrease in recurrent PTB at <37 weeks of gestation compared to placebo in women with singleton pregnancies and a history of SPTB at 20-36 6/7 weeks of gestation. Progesterone was also found to positively correlate with significant decreases in both overall PTB and neonatal complications.30
The OPPTIMUM trial found that the use of vaginal progesterone in women with a history of SPTB was not associated with a statistically significant decrease in the risk of PTB, nor did it affect the rate perinatal mortality and morbidity (progesterone 15.9% vs. placebo 18.8%).11
Three studies that directly compared 17-OHPC and vaginal progesterone in women with prior SPTB have been published in the literature.33-35 Saccone et al36 included these studies in a meta-analysis which consisted of 680 women in total. The largest trial, conducted in Saudi Arabia, accounted for 74% of subjects in the meta-analysis.35 They concluded that in women with singleton pregnancies and a history of SPTB, a daily vaginal progesterone can be used as an alternative to weekly 17-OHPC injection for SPTB prevention. The significant heterogeneity between study findings, attributed to the multifactorial origin of SPTB, should be taken into consideration when interpreting such data. Some studies have also examined the role of vaginal progesterone in this setting35,37 while others used 17-OHPC.30,38
A double-blind, placebo-controlled, international (PROLONG) trial published in 2019 found no benefit of weekly IM injection of 250 mg of 17-OHPC (from 16 to 36 weeks of gestation) compared to placebo in decreasing recurrent PTB risk and neonatal morbidity when given to women with singleton gestation and a history of SPTB.39
In women with a singleton gestations and a history of SPTB at 20-36 6/7 weeks of gestation, the use of 17-OHPC 250 mg intramuscular weekly injection, starting at 16-24 weeks until 36 weeks of gestation is recommended (Grade 2B).
Vaginal progesterone preparations may be considered as an alternative if 17-OHPC is not available for this group of women (Grade 2C).
What is the role of progesterone in singleton pregnancies with a history of PTB and short CL?
In women with singleton pregnancies, short cervix (TVU CL <15 mm) and a history of PTB, Fonseca et al40 compared vaginal progesterone to placebo and found no statistical significant difference in the SPTB rate at <34 weeks of gestation. Similarly, in 2011, the PREGNANT trial by Hassan et al41 found that in women with a history of PTB and TVU CL <20 mm, vaginal progesterone was not linked to a significantly lower rate of PTB. Moreover, in a subgroup analysis of a meta-analysis by Romero et al,42 the beneficial effect of vaginal progesterone did not significantly differ between women with a short cervix and a history of SPTB and those without a prior SPTB. Berghella et al42 conducted a trial that failed to demonstrate any benefit of 17-OHPC in women with a history of SPTB and a cerclage for short cervix (TVU CL <25 mm). Although, in women without a cerclage 17-OHPC appears to play a role in reducing pre-viable birth and perinatal mortality.43
In a study by Conde-Agudelo et al44 evaluating both interventions including vaginal progesterone and cervical cerclage, the effectiveness of both interventions was similar in reducing the risk of SPTB and perinatal adverse outcome in women with a mid-trimester sonographic short cervix, singleton gestation, and prior PTB.
In women with a prior SPTB who began the 17-OHPC treatment, the continuation of 17-OHPC therapy throughout pregnancy, regardless of the cervical length (with or without placement of cervical cerclage when indicated), is recommended (Grade 2B).
In women with a previous SPTB and TVU CL <25 mm as detected on a transvaginal ultrasound at 18-24 weeks of gestation, a daily administration of 200 mg of vaginal progesterone is recommended. That is appropriate only if the patients have not yet commenced 17-OHPC treatment (Grade 2B).
What is the role of progesterone in twins and higher-order multiple pregnancies?
Randomized trials of 17-OHPC to primarily prevent PTB in twin pregnancy with unknown CL have not established its efficacy to prevent PTB or reduce overall twin morbidity resulting from prematurity.45,46 The PROGESTWIN study showed that intramuscular 17-OHPC therapy did not reduce PTB before 37 weeks in unselected twin pregnancies. Nevertheless, intramuscular 17-OHPC significantly decreased neonatal morbidity and increased birth weight.47
The use of 17-OHPC in unselected triplet pregnancies did not result in a statistically significant difference in the rate of SPTB in the 17OHPC group compared to the placebo group.48,49 This finding was observed regardless of history of PTB, method of conception, or type of chorionicity. Similarly, 17-OHPC did not reduce PTB in twin pregnancies with sonographic short cervix (TVU CL <25 mm).50
A randomized controlled trial by Senat et al51 showed that increasing progesterone dose for women with twin pregnancy was linked to a statistically significant increase in early PTB at <32 weeks of gestation in the intent-to-treat analysis (29% treated vs. 12% control, 2.5-fold increase, p=0.007).51 Additionally, the treated group observed a statistically substantial increase in the rate of perinatal mortality and a composite adverse outcome of stillbirth and respiratory distress.52,53
Studies on vaginal progesterone use in twin pregnancy failed to show a significant decrease in PTB risk, neonatal mortality, and morbidity.54-56 One study showed that when used for mid-trimester short cervix in twin pregnancies, vaginal progesterone does not lower the rate of PTB compared to placebo.57 However, in a meta-analysis by Romero and colleagues, the use of vaginal progesterone in women with mid-trimester sonographic short cervix (TVU CL <25 mm) did not positively impact PTB rate before 33 weeks’ gestation, but was associated with a 48% decrease in neonatal mortality and complications. It also showed a drop in PTB risk in women <37 weeks’ gestation with a twin pregnancy and no prior PTB.58-60
Romero et al61 demonstrated, in an updated individual patient data meta-analysis, a significant reduction of both PTB risk at <33 weeks of gestation by 31% and neonatal death by 47% when vaginal progesterone administered to women with a twin pregnancy and an asymptomatic mid-trimester sonographic short cervix (TVU CL <25 mm). Moreover, vaginal progesterone group was found to have a significant reduction in global SPTB risk, perinatal death, and composite neonatal adverse outcome.61
In women with multiple gestations, prophylactic progesterone is ineffective in PTB prevention or reduction of overall twin morbidity (Grade 1B).
Increasing the 17-OHPC dose for women with twin pregnancy may be harmful (Grade 2C).
Women with twin pregnancy and sonographic short cervix treated with vaginal progesterone had a nominal drop in PTB risk at <33 weeks and a significant reducing in composite neonatal adverse outcome (Grade 1B).
There is limited information regarding the use of progesterone to prevent the recurrence of PTB in multiple pregnancies where there is a prior history of SPTB.
What is the role of progesterone in patients with preterm premature rupture of membranes (PPROM)?
Progesterone supplementation exhibits no benefit in the prevention of SPTB in women with PPROM. A large meta-analysis failed to demonstrate a prolonged latency period or improvement in maternal or neonatal outcomes.62
In women with PPROM, progesterone has not demonstrated any beneficial effect and is, therefore, not recommended (Grade 1A).
What is the role of progesterone in patients present with preterm labor?
A Cochrane systematic review of eight trials concluded that there is not enough evidence to encourage the use of progesterone to prevent PTB in women with threatened or experienced preterm labor.63 However, some data suggest that the use of progesterone reduced preterm deliveries <37 weeks of gestation and increased birthweight. Nevertheless, due to the relatively small number of available studies, the analysis was limited. The varying types, doses and administration routes of progesterone also limited the power of the meta-analysis.
There is currently not enough evidence to recommend progesterone use to prevent PTB in pregnancies complicated with PPROM or threatened/established PTL.
What are the optimal doses and the ideal timing for the initiation and cessation of progesterone in the prevention of PTB?
PTB prevention trials include the use of a variety of progesterone doses. Based on the US datasets, 250 mg 17-OHPC is predominantly administered weekly via intramuscular injection.36 Vaginal progesterone is also available and may have the advantage of fewer systemic side effects, although reports of vaginal irritation have arisen due to this route. Nonetheless, doses of 90-400 mg have been used through this route of administration, but the optimal dosage has not been established. A subgroup analysis of the recent meta-analysis in 2018 by Romero showed no difference in effect between 90-100 mg and 200 mg/d of vaginal progesterone for women with a short cervix.42
The timing of therapy varied between studies and began as early as week 16 of gestation in women with a previous history of PTB. In some trials, treatment began between weeks 16-24 of pregnancy when an ultrasound scan diagnosed the short cervix. Other studies show no difference in the efficacy of progesterone therapy when initiated at 16-20 weeks compared to 20-26 weeks.64,65 Rebarber found in a retrospective analysis that early interruption of 17-OHPC therapy has been shown to increase spontaneous recurrent PTB risk.66 Therefore, continued treatment until 36-37 weeks of gestation is recommended.
Progesterone therapy should begin at 16-24 weeks of gestation when indicated and continue until 36-37 weeks or delivery, whichever occurs first.
Summary of recommendations and level of evidence:
Footnotes
Disclosure. Authors have no conflict of interests and the work was not supported or funded by any drug company.
- Received July 30, 2019.
- Accepted February 4, 2020.
- Copyright: © Saudi Medical Journal
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.






