Abstract
Host immune response to coronaviruses and the role of cross-reactivity immunity among different coronaviruses are crucial for understanding and combating the continuing COVID-19 outbreak and potential subsequent pandemics. This review paper explores how previous exposure to common cold coronaviruses and more pathogenic coronaviruses may elicit a protective immune response against SARS-CoV-2 infection, and discusses the challenges posed by some variants of concern that may escape current vaccines. It also highlights the need for a mucosal universal vaccine that can induce long-term protection against current and emerging coronaviruses by leveraging cross-reactive immunity. We propose a novel mucosal universal vaccine that consists of cross-reactive antigenic peptides with highly conserved epitopes among coronaviruses, conjugated with an immunostimulant adjuvant cytokine, including B-cell activating factor (BAFF). This vaccine may enhance the local mucosal adaptive response, induce tissue-resident memory cells, and inhibit viral replication and clearance. However, further research is required to evaluate its safety and efficacy.
Coronaviruses are a large family of ribonucleic acid (RNA) viruses that can cause diseases in both humans and animals, and have caused significant public health concerns, and some human coronaviruses, such as OC43, NL63, 229E, and HKU1, can cause mild to moderate flu-like symptoms.1,2 However, over the past twenty years, the world has faced 3 major pandemics of serious and lethal human coronaviruses, namely Severe Acute Respiratory Syndrome coronavirus (SARS-CoV) in 2003, Middle East respiratory syndrome coronavirus (MERS-CoV) in 2012, and the new coronavirus (SARS-CoV-2) which was found at the end of 2019 and has caused the ongoing outbreak of COVID-19 disease.2-4
The appearances of these viruses have encouraged us to achieve a deeper understanding of their disease mechanisms, transmission modes, and host immune interactions, and particularly the role of cross-reactivity among them. Furthermore, despite their distinct nature, these coronaviruses share several similarities in their genomic structure, receptor usage, and clinical manifestation, and multiple studies have provided evidence that the sera obtained from people who are infected with MERS-CoV or SARS-CoV have the ability to cross-react with SARS-CoV-2, and vice versa.5-11 This cross-reactivity is thought to be caused by the high resemblance in the genes among the viruses.
As a result of the COVID-19 outbreak, large numbers of people globally have received vaccinations from diverse vaccine platforms to prevent the outbreak of COVID-19 disease, resulting in the survival of more than 20 million people. However, the effectiveness of the original vaccines has notably diminished because of the emergence of significant SARS-CoV-2 variants of concern.12 The majority of currently approved vaccinations are injected and stimulate the creation of specific immunoglobulin G (IgG) responses that can deactivate SARS-CoV-2 and reduce COVID-19 symptoms. Nevertheless, they have limited capacity to induce mucosal immunity.13 Therefore, designing an efficient mucosal and universal vaccine that is administered directly through the mucosal route may strengthen and increase the pulmonary immune response and thus reduce viral replication and release into the airways. Cytokines, such as B-cell activation factor (BAFF), which can directly induce a B-cell response, antibody production and memory response, may be used as mucosal vaccine adjuvants against SARS-CoV-2 infection, as we have recently reported.14,15
Ultimately, a better understanding of the role of cross-reactivity between coronaviruses is crucial for the development of diagnostic tools and treatments, and more importantly, for designing effective vaccines, which can offer broad-spectrum activity and protection against multiple emerging coronaviruses. In this review, we present a summary of the current knowledge of the role of cross-reactivity between the circulating common cold coronaviruses, as well as MERS-CoV and SARS-CoV, and their possible protection against SARS-CoV-2 infection. In addition, we consider the implications of cross-reactivity in developing universal mucosal adjuvant vaccines that may provide broad protection against emerging coronaviruses.
The similarities and differences between epidemic and pandemic human coronaviruses
Human coronaviruses can be categorized as either epidemic or pandemic. Epidemic coronaviruses cause regional outbreaks, whereas pandemic coronaviruses cause global outbreaks.16 Epidemic and pandemic coronaviruses are members of the family Coronaviridae and both types have the ability to cause respiratory illnesses, and share a similar mode of transmission and genetic structure. They are transmitted by respiratory droplets and can cause fever, coughing, and shortness of breath.16 The key differences between epidemic and pandemic human coronaviruses can be distinguished by severity and global impact.16 Epidemic coronaviruses, such as human coronavirus NL63, HKU1, OC43, HKU1, and 229E, usually cause mild respiratory infections and are not linked with major morbidity or mortality.16 However, the pandemic coronaviruses, including MERS-CoV, SARS-CoV, and SARS-CoV-2, have significant consequences for public health as well as for the economy.17 Although these viruses share some similarities, they differ significantly in their genetic makeup and virulence. In addition, the disease transmission differs, and in comparison to SARS-CoV, SARS-CoV-2 is very contagious.18,19 Additionally, the severity of infections caused by these viruses varies greatly, with SARS-CoV and MERS-CoV causing markedly fewer epidemics, but with extremely high mortality rates. The mortality rates for SARS-CoV is 10% and 37% for MERS-CoV, while SARS-CoV-2 has caused a global outbreak, but with a comparatively lower case fatality rate.20,17 However, the immune response to these viruses is quite similar.21 The angiotensin-converting enzyme 2 (ACE2) is shared receptor between SARS-CoV and SARS-CoV-2 to enter human cells, whereas MERS-CoV enters the host cells via dipeptidyl peptidase 4 (DPP4).3 Overall, epidemic and emerging coronaviruses have several commonalities, including their potential to cause respiratory illnesses, their origin from animals, and their ability to lead to epidemics or pandemics. These viruses are primarily transmitted through zoonotic events, often from wild animals.17,22 Therefore, genetic homology among these viruses may increase chances of immune cross-reactivity with each other and offer some protection.
Role of cross-reactivity in providing protection among coronaviruses
Several studies have suggested that previous infection with circulating coronaviruses may provide some immunological protection against SARS-CoV-2, but the duration of this protection is still unknown.23-26 Furthermore, it has been found that memory T-cells can cross-react and mediate protection against COVID-19.27,28 However, other studies have reported that prior infection with circulating coronaviruses may not play a role in providing protection against subsequent SARS-CoV-2 infection or even modulate disease severity.28 Moreover, memory B-cells have been observed to mediate cross-reactivity to the SARS-CoV-2 spike protein (S2) subunit during infection.29 In addition, cross-reactive antibodies have also been shown to bind to the S2 of SARS-CoV-2, as well as other coronaviruses.30,31 Despite these findings, it has been shown that exposure to seasonal coronaviruses does not confer immunological protection against SARS-CoV-2.32 It is important to note that the extent of cross-reactivity protection may vary among individuals’ immune systems, as well as the specific pre-existing immunity to certain strains of coronaviruses.33 Collectively, these observations highlight the importance of observing the level and durability of protection against SARS-CoV-2, and how this could enhance vaccine development. Thus, further investigations are required in order to understand the nature and length of this protection. Interestingly, earlier in the COVID-19 pandemic, it was found that normal blood donors had a strong and lasting T-cell immunity against SARS-CoV-2. These T-cells could also recognize and respond to other seasonal epidemic coronaviruses, such as OC43, HKU1, 229E, and NL63, and this might be because of previous infections with these viruses.28 Additionally, it has been demonstrated that mice infected with SARS-CoV-2 can be protected from infection by the SASR-CoV-1 vaccine.34 Collectively, these outcomes convey the first evidence that coronavirus vaccines can provide protection against heterologous coronaviruses, accordingly supporting the development of universal coronavirus vaccines.
Impact of cross-reactivity on diagnosis rapid test and vaccinations
Cross-reactivity amongst coronaviruses presents a challenge in developing rapid detection kits, and false positive and negative readings may result in unnecessary procedures such as quarantine, treatments, and spread of the virus.35 Cross-reactivity amongst coronaviruses has been implicated to cause incorrect results. For instance, specific antibodies that target the SARS-coronavirus nucleocapsid protein were found to be cross-reactive with human coronaviruses 229E and OC43.36 Additionally, it has been reported that there is cross-reactivity between dengue virus (DENV) and SARS-CoV-2 IgG antibodies.37 Therefore, in order to overcome these issues, rapid detection methods must minimize the risk of cross-reactivity and exhibit high sensitivity and specificity.38 Nanobiosensors have been proposed as a simple and quick diagnostic platform for use in the early stages of the disease, but their accuracy, specificity, and sensitivity need to be improved.39 Thus, to avoid inaccurate results, additional investigations are required to completely comprehend the implications of cross-reactivity among coronaviruses in the development of detection kits. In addition, it has been demonstrated that previous immunity to seasonal human coronaviruses can influence the magnitude and cross-reactivity of the antibody response to SARS-CoV-2 vaccination.40 Moreover, the activation of previously cross-reactive memory B-cells during SARS-CoV-2 infection indicates that previous responses may influence the SARS-CoV-2 infection.41 However, the impact of cross-reactive immunity on the efficacy of SARS-CoV-2 vaccination is not yet completely understood.26 Although cross-reactive antibodies may enhance the effectiveness of vaccines, cross-reactivity may reduce vaccine effectiveness.42 The effect of cross-reactivity immunity on SARS-CoV-2 vaccination may depend on the specific epitopes targeted by the vaccine and the degree of similarity between the vaccine and pre-existing coronaviruses.26 Consequently, considering the potential impact of cross-reactivity is crucial while creating and assessing vaccines for SARS-CoV-2.
Implication of cross-reactivity in the development of a universal mucosal vaccine against coronaviruses
The COVID-19 outbreak underscored the need to quickly create and distribute efficient vaccines to control the spread of infectious diseases. Additionally, the appearance of new variants of SARS-CoV-2 has reduced the efficiency of vaccinations and natural immunity, which is one of the current difficulties in containing the COVID-19 outbreak.43 The rapid waning of coronavirus immunity can be considered as another challenge to the design of optimal coronavirus vaccines.44 The high mutation rate of SARS-CoV-2 may also lead to reductions in the effectiveness of vaccines as a result of the generation of multi-neutralizing epitopes.43 Additionally, a diverse range of coronaviruses has been shown in different cell lines and animals.45,46 The SARS-CoV-2 spike, for example, may bind ACE2 relatives from domestic animals and initiate viral entry, which may suggest that other coronaviruses, particularly SARS coronaviruses, which originated from bats, are potentially spreading from animals to humans more frequently and may cause another pandemic.43,46,47 Consequently, the establishment of a universal coronavirus vaccine that is capable of offering lifelong defence against a variety of coronavirus strains, including recently re-emerging as well as emerging variants of concern, is urgent. Moreover, targeting SARS-CoV-2 and SARS-CoV’s conserved antigenic areas has been found to be effective and has the ability to induce robust antibodies that can neutralize multiple variants of concern, as these antigenic sites remain largely consistent across many strains.48
Moreover, identification and deeper understanding of cross-reactive antibody sites on the viral genome among more pathogenic coronaviruses can afford essential information for the development of effective vaccines and therapeutics for a wide range of deadly coronaviruses, which is critical for dealing with recent and possible future outbreaks. Certain antibodies produced by naive B-cells have been demonstrated to neutralize numerous coronaviruses, such as SARS-CoV, SARS-CoV-2, and SARS-WIV1-CoV and its variants, by identifying specific areas of the viral receptor-binding domain (RBD).49 Currently, efforts are ongoing to develop an effective vaccine that can afford protection against SARS-CoV-2 in addition to other potential emerging coronaviruses.
Mucosal vaccines are a promising technique because they can elicit immune responses at the mucosal surfaces through which the virus enters.50 In addition, animal studies have demonstrated that mucosal immunization provides long-lasting and broad protection against related coronaviruses including SARS-CoV and MERS-CoV.51 Furthermore, mucosal vaccinations can generate antibodies that neutralize the virus in the mucosa, preventing SARS-CoV-2 airborne transmission.52 Additionally, a recent study that used a plasmid vaccine that employs quil-a-loaded chitosan nanoparticles as mucosal adjuvant has shown robust immune response against avian coronaviruses.53 Another study has found that an mRNA vaccine that encodes multiple coronavirus peptides from different strains can provide cross-protection against various coronaviruses.54
Moreover, a current study showed that the EpiVacCorona vaccine in animal models can induce effective immunity against SARS-CoV-2 and is able to cross-react with heterologous coronaviruses and enhance immune protection.55 In addition, it has been shown recently that a universal mRNA multi-epitope vaccine, which uses conserved T-cell and B-cell epitopes from the influenza virus, may offer potential protection against various subtypes of influenza strains A and B.56-58 This universal flu vaccine may reduce the yearly flu impact caused by different strains of influenza B viruses that circulate together. Similarly, a universal vaccine against pan-coronaviruses that can provide broad protection against potential future outbreaks could utilize an mRNA multi-epitope vaccine targeting highly conserved regions shared among coronaviruses.
From the above, we can conclude that a level of protection from SASR-CoV-2 infection is offered from the pre-existing immunity generated from previous coronavirus infections, and that this has led to improvements to the efficiency of current vaccinations for COVID-19. Thus, these observations could suggest that developing one vaccine against a certain strain of coronavirus can be effective against other coronaviruses as a result of cross-reactivity among these viruses, therefore establishing the possibility of developing a universal coronavirus vaccine that can provide broad protection against epidemic and emerging viruses that may cause potential outbreaks in the future. To achieve this goal, it is very important to determine the shared and conserved epitopes among coronaviruses. The SARS-CoV-2 functional and structural proteins, including S, N, and ORF1ab, have the most promising cross-reactivity, especially the S2 part of the spike protein, which has many epitopes that can react with various antibodies. However, the immune responses to ORF1ab epitopes seems to be very specific for each individual.59 Thus, targeting conserved regions of the virus that are shared by multiple strains can be achieved through a universal vaccination that may offer protection against a broad range of coronaviruses and help to prevent future pandemics.
A recent immunoinformatics study has investigated the common epitopes shared among human and animal coronaviruses and has identified a wide range of human B- and T-cell epitopes, with high similarity among the epitope sequences of viruses such as SARS-CoV-2, SARS-CoV, and bat-SL-CoV.60 These include CD8+ T cell epitopes, which were able to cross-react among SARS-CoV and SARS-CoV-2: ORF1ab2363–2371, ORF1ab3013–3021, S958–966, and S1220–1228. Similarly, a B-cell epitope (S287–317) has been described as cross-reactive among SARS-CoV and SARS-CoV-2.61
Furthermore, the spike (S) protein of SARS-CoV-2 is one of the conserved immunodominant antigens, and it is considered to be the main target for the neutralizing antibodies as well as vaccines, and interestingly, SARS-CoV-2 variants have contained a majority of the mutations, whereas the N protein appears to exhibit high levels of similarity across various lineages and variants.61 Besides, another study has identified two conserved B-cell epitopes in the N protein of SARS-CoV-2, namely N 185–197 and N 277–287, which are highly similar in main variants and cross-react with samples from COVID-19 from recovered patients and in MERS-CoV.62 Therefore, the highly conserved human B- and T-cell epitopes may have huge implications for the creation of a universal preventive vaccine to induce an effective immune response against all coronaviruses.
Additionally, the majority of vaccinations that are currently used are given by injection and can induce the generation of specific IgG responses that can block SARS-CoV-2 and reduce COVID-19 symptoms. However, they have a limited capacity to induce mucosal immunity.13 Mucosal adjuvants have the ability to activate the immune system at mucosal surfaces, which are the main sites of pathogen entrance and reproduction.63
Cytokines that induce a B-cell response and antibody production, as well as generating long-lasting tissue resident B-cells in the airways, such as B-cell activation factor (BAFF), can activate lung B-cell responses and antibody production during viral respiratory infections.14 BAFF plasma levels were significantly increased in lung of infected mice with RSV compered to control animals.64 Moreover, BAFF levels were higher in non-intensive care unit (ICU) COVID-19 patients than in ICU and healthy controls.15 However, another study reported that BAFF levels were increased significantly in sever COVID-19 patients than in the mild group, indication the strong B cells response.65 Together, we believe that BAFF can support local B cells response and antibody production in the airways and therefore can offer a strong local airways immune repose against pulmonary viral infection, and hence BAFF can be used as mucosal immunostimulant adjuvant against SARS-CoV-2 infection, as we have recently reported.14
Collectively, we suggest that synthesis of antigenic peptides with multi-epitopes that are shared and cross-reactive with various coronaviruses covering the following B- and T-cell epitopes – ORF1ab2363–2371, ORF1ab3013–3021, S958–966, and S1220–1228, S287–317, N185–197 and N 277–287 – conjugating with BAFF cytokines, may induce a robust immune response at the mucosa and long-lasting tissue resident memory cells, and thus provide broad protection against current and future coronavirus outbreaks (Figure 1). However, overexpression or dysregulation of BAFF cytokine expression has been linked with the onset of autoimmune disorders, and thus further research is required to ensure the safety and efficacy of the suggested vaccine.66,67
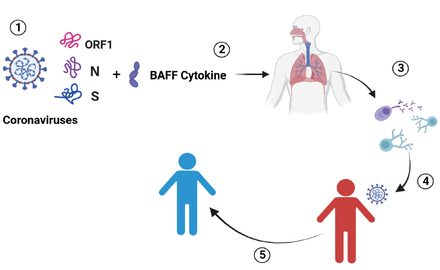
- Proposed a universal adjuvant mucosal vaccine against coronaviruses infection: As the main entry point for respiratory viral infection is the mucosal surfaces, thus, targeting them can contribute to overcome early viral respiratory infection. Herewith, the most common conserved shared epitopes among circulating and more pathogenic coronaviruses were identified and used them with an antigenic peptide linked with the immunostimulant cytokine B-cell activation factor (1). This vaccine is delivered through respiratory system, such as nasal route (2). This vaccine may increase the local adaptive host immune response, including cellular and humoral immune responses and the production of long-life memory cells in the mucosa (3). After coronaviruses infection (4), this vaccine may prevent early viral replication and thus, offer protection (5).
In concluaion, the current evidence suggests that the similarity among coronaviruses can cause cross- reactivity amongst them and this may decrease the potential infection with SARS-CoV-2. This cross-reactivity may also affect the results from rapid detection commercial kits and cause false readings. However, the magnitude and durability of cross-reactive immunity are as yet unknown, and additional studies are required to determine its potential impact on vaccine efficacy in the long term. Moreover, it is essential to enhance our knowledge of the fundamental process and identify specific cross-reactive and highly conserved epitopes among coronaviruses in order to develop accurate viral detection kits, effective treatments, and a pan-coronavaccine that can provide wide immunity against new coronaviruses in the future.
Finally, we suggest a novel mucosal universal antigenic peptide conjugated with a BAFF cytokine adjuvant that utilizes the highly conserved multi-epitopes among coronaviruses against past, current and potential coronavirus outbreaks. These multi-epitopes have been revealed to elicit strong and effective adaptive immune responses against coronaviruses, including T- and B-cell effector functions. Furthermore, as the route of virus infection is the respiratory tract system, a mucosal vaccine may induce a more effective immune response than a systematic vaccine. Thus, administration of the proposed vaccine via the mucosal surfaces may be able to initiate an effective local immune response as well as generating long-lasting tissue-resident memory cells. Nevertheless, overexpression of BAFF cytokines has been associated in the onset of autoimmune diseases: therefore, additional studies should be carried out to ensure the safety and efficacy of the proposed vaccine.
Acknowledgment
I would like to the thank the deanship of scientific research at Majmaah University of supporting this study under project number (R-2023-525). I would like to thank Essay Doctor Academic Proofreading (www,academicproofreading.co.uk) for the English language editing.
Footnotes
Disclosure. Authors have no conflict of interests, and the work was not supported or funded by any drug company.
- Copyright: © Saudi Medical Journal
This is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.