Abstract
Objectives: To estimate the incidence and prevalence of prostate cancer in Saudi Arabia.
Methods: This is a retrospective cohort study including male patients aged 40 years and over. The prostate-specific antigen screening tests were carried out in a community-based clinic affiliated with King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia between September 2002 and December 2016.
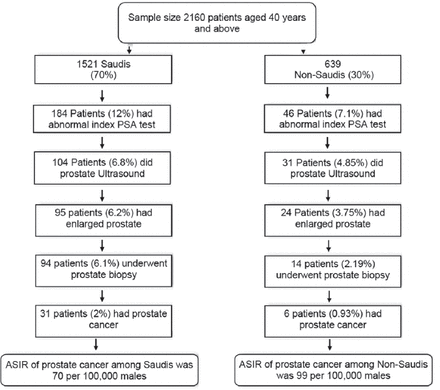
Results: A total of 2,160 male patients were included in the study. Of these, 1,521 (70%) were Saudi nationals and 639 (30%) were non-Saudi nationals. A total of 108 (5%) patients underwent a prostate biopsy. The biopsy results confirmed that 31 (2%) Saudi patients and 6 (0.93%) non-Saudi patients had prostate adenocarcinoma. The age-standardized incidence rate of prostate cancer in the Saudi male population is 70 per 100,000 males. Nearly two-thirds (71%) of the Saudi patients’ prostate cancer was found to be in the early stages.
Conclusion: The prevalence of prostate cancer in the Saudi male population is higher than that reported by the Saudi Cancer Registry; however, it is low compared with prevalences in developed countries. The mortality rate is also very low. Prostate-specific antigen screening in Saudi Arabia should not be carried out routinely; instead, it should only be carried out on an individual basis.
Prostate cancer, one of the most common malignancies affecting men, represents 21% of all cancer incidences. It is also the second leading cause (8%) of male cancer-related deaths in the United States.1 Furthermore, prostate cancer is the fifth leading cause of cancer-related deaths in men worldwide, which accounts for 6.6% of all deaths in men.2
Data from the 2014 Saudi Cancer Registry report3 showed that prostate cancer was ranked as the fifth leading cause of cancer in men and constitutes 6.1% (324 cases) of total cancers in men in Saudi Arabia. The overall age-standardized incidence rate (ASIR) of prostate cancer in Saudi Arabia between 2001 and 2008 was estimated to be 5.1 per 100,000 males. However, these figures come from an analysis of the Saudi Cancer Registry4 and, likely due to poor reporting, represent an underestimation of the epidemiology of the disease.5 This could explain the low reported incidence of prostate cancer in Saudi Arabia compared with other countries.
The American Urological Association (AUA) recommended the use of prostate-specific antigen (PSA) screening for prostate cancer among men aged between 55 and 69 years after shared decision-making. They recommended the screening of men aged between 40 and 54 years who are at high risk, such as African American and those with first-degree relatives affected by the disease.6 In contrast, other agencies, such as the US Preventive Services Task Force (USPSTF), recommends against the use of routine PSA screening.7 Similarly, the Canadian Task Force on Preventive Health Care recommends against the use of PSA testing for prostate cancer screening.8 The European Association of Urology recommends that men should not be subjected to PSA testing without counseling on the pros and cons of the screening. Screening should be offered to men at high risk who are well informed, have a good performance status, and have a life expectancy of at least 10-15 years.9
Studies evaluating the yield of PSA screening in Saudi Arabia are lacking. We conducted this community-based study aiming at estimating the incidence and prevalence of prostate cancer in Saudi Arabia.
Methods
Medical health records of asymptomatic patients seen in family medicine clinics linked to the King Faisal Specialist Hospital and Research Centre (KFSH&RC) were retrospectively reviewed. We collected demographic data and laboratory results, including PSA test results, prostate volume, numbers of prostate biopsies, diagnoses of prostate cancer with Disclosure. Authors have no conflict of interests, and the work was not supported or funded by any drug company. grade and stage, date of diagnosis, and treatment options that the patients underwent. The patients were followed from their index PSA test, the first PSA screening test.
The inclusion criteria of our study included all the results of asymptomatic male patients aged 40 years and over who underwent PSA screening tests in family medicine clinics linked to the KFSH&RC between September 2002 and December 2016.
All patients who were already diagnosed with prostate cancer or those who had prostate symptoms were excluded from the study.
Statistical analysis
Data analyses were conducted using the Statistical Package for Social Science, version 25 (IBM Corp., Armonk, NY, USA). Descriptive statistics, mean and median, were used for continuous variables, and categorical variables were summarized as frequencies and percentages. Regression analysis was used to assess the relationship between an outcome variable and a risk factor. The incidence rate was calculated by dividing the total number of newly diagnosed prostate cancer patients by the total person-years-at-risk. We found the person-years-at risk by calculating the time difference between the date of the index PSA screening test and the date of either diagnosis, death, or the last follow-up. The overall ASIR was calculated from Segi’s10 world standard population. The level of statistical significance was set at p<0.05. The research project was ethically approved by the Research Advisory Committee of the KFSH&RC.
A 95% confidence interval (CI) was used to control the error of estimation to be within a 3% error margin. The estimated sample size was 1,067 subjects. However, the study included all asymptomatic male patients.
Results
A total of 2,160 male patients were included in the study. Of these, 1,521 (70%) were Saudi nationals and 639 (30%) were non-Saudi nationals. This ratio of Saudi to non-Saudi males in the sample was similar to the ratio of Saudi and non-Saudi males aged 40 and over in the general population.11 The mean age was 61 years (95% CI: 60.4-61.5). The mean PSA level was 2.5 ng/mL (95% CI: 2.1-2.8) and the median was 1.1 ng/mL (95% CI: 0-152.1). The mean BMI was 28.4 (95% CI: 28.2-28.6) (Table 1).
The overall mean and median for different variables of the study.
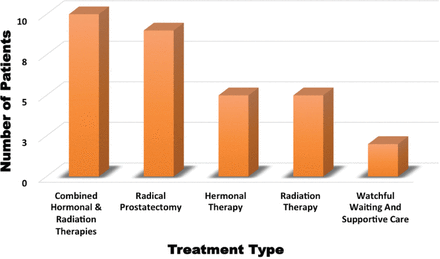
Out of 1,521 Saudi patients, 184 (12%) had abnormal index PSA tests. Of these, 104 patients (6.8%) received a prostate ultrasound. Ninety-five patients (6.2%) had enlarged prostates and, subsequently, 94 patients (6.1%) underwent a prostate biopsy. Of those who had a prostate biopsy, 31 (2%) had adenocarcinoma. The ASIR of prostate cancer among Saudi males from September 2002 and December 2016 was 70 per 100,000 (Figure 1). Among those diagnosed with prostate cancer, 12 patients (39%) had Gleason scores of ≤6 on diagnosis, whereas 19 patients (61%) had Gleason scores of 7-10. Most of the patients (22) diagnosed with prostate cancer (71%) were in the early stages; however, 9 patients (29%) had advanced prostate cancer. Hormonal therapy was given to 15 patients, out of which 10 patients also received supplemental radiotherapy. Nine patients had a radical prostatectomy and 5 patients received radiotherapy alone. Watchful waiting and supportive care were adopted for 2 patients (Figure 2). One patient with prostate cancer died due to other comorbidities.
The summary of the study of 2,160 patients who underwent prostate-specific antigen.
Treatment options provided for prostate cancer patients in the study.
Out of the 639 non-Saudi patients, 46 (7.1%) had an abnormal index PSA test. Of these, 31 patients (4.8%) had a prostate ultrasound and 24 (3.7%) were found to have an enlarged prostate. Prostate biopsies were carried out on 14 patients (2.1%), and prostate adenocarcinoma was confirmed in 6 patients (0.9%). The ASIR of prostate cancer among non-Saudis in the Saudi Arabian male population from September 2002 to December 2016 was 99 per 100,000 (Figure 1). Among those diagnosed with prostate cancer, 5 patients (83.3%) had Gleason scores of ≤6 on diagnosis, whereas one patient (16.6%) had a Gleason score of 7-10. Most of the patients (5; 83.3%) were in the early stages; however, one patient (16.6%) had advanced stage cancer. Two patients had radical prostatectomies, and 2 patients received combined hormonal therapy and radiotherapy. One patient received hormonal therapy alone, and one patient received no treatment and left the country.
Discussion
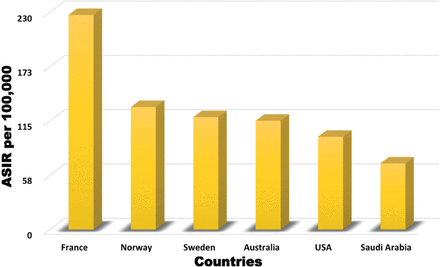
The ASIR of prostate cancer among the included Saudi male population from September 2002 to December 2016 was 70 per 100,000, which is higher than that reported from the hospital-based Saudi Cancer Registry.3 This can be explained by the low detection rate and the low level of reporting of prostate cancer cases, especially from peripheral parts of the country.5 Primary healthcare centers in Saudi Arabia are, in general, not well equipped to screen for prostate cancer, and PSA tests are not available in most of these centers. Alternatively, family medicine clinics linked to the KFSH&RC have access to all the facilities available at the KFSH&RC, including PSA tests. The ASIR of prostate cancer reported in our study is still in the lower range reported by other countries, such as France (227), Norway (129), Sweden (119), Australia (115), and the United States (98) per 100,000 men (Figure 3).12
The age standardized incidence rate of prostate cancer compared to other countries.
The index PSA test was found to be abnormal in 230 patients (11%). This is close to the finding (11.9%) of Walter et al.13 The percentage of patients who underwent a prostate biopsy in our study was 5% of the total sample (2,160), and 1.7% were diagnosed with prostate cancer. This is close to the finding of Hoffman et al,14 where 6.3% of 2,620 screened patients underwent a biopsy and 2.3% were diagnosed with prostate cancer. The mean age at the time of diagnosis of prostate cancer in our study was 61 years, which is close to the finding of Zahir et al,15 who reported that the mean age of the diagnosis of prostate cancer was 67 years. Our results showed that 17 patients (46%) had Gleason scores of ≤6 on diagnosis, whereas 20 patients (54%) had Gleason scores of 7-10. This is close to the finding of Zahir et al,15 who reported that 31% of prostate cancer patients were found to have low to moderate Gleason scores (≤6) and 69% were found to have high Gleason scores (7-10).
Age over 60 years was associated with an increased incidence of prostate cancer, with a relative risk of 3.00 (95% CI: 1.50–6.02), which is a well-known risk factor for the disease.16-17 There was no association between obesity (BMI ≥ 30) and the incidence of prostate cancer in our study (relative risk = 1.05: 95% CI: 0.51–2.14). This is a recently controversial area of research because findings of an association between obesity and prostate cancer are contradictory and no association has yet been established.18
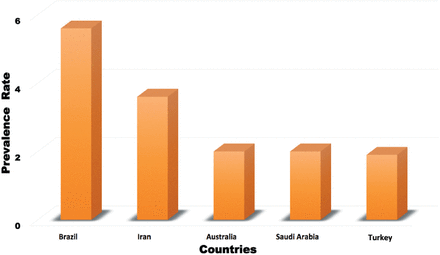
In our study, the prevalence of prostate cancer among Saudis was 2%, which is in the lower range of the prevalence of prostate cancer in other regional and international countries, such as Brazil (5.6%), Iran (3.6%), Australia (2%), and Turkey (1.9%) (Figure 4).19-22
The prevalence of prostate cancer compared to other countries.
The prevalence and incidence of prostate cancer in our study was in the lower range compared with the other countries.12,19-22 In addition, prostate cancer mortality in our study population was very low. Therefore, we do not recommend routine PSA screening in Saudi Arabia, which is in line with most international guidelines, such as the USPSTF.7,8
Study limitations
This is a community-based study to evaluate the incidence and prevalence of prostate cancer in Saudi Arabia. The PSA testing was undertaken at the KFSH&RC laboratory, which is accredited by the College of American Pathologist’s laboratory accreditation program and joint commission accreditation. One of the limitations of this study is that it was a retrospective study conducted in family medicine clinics affiliated with KFSH&RC; however, the demographics of the sample were similar to the demographics of the general population of Saudi Arabia. A larger prospective community-based study covering the populations of different regions of Saudi Arabia is recommended.
In conclusion, the prevalence of prostate cancer in this study’s population was higher than that reported by the Saudi Cancer Registry; however, it was lower than the prevalence reported by several other countries. Prostate cancer screening is associated with potential harms and costs and the mortality rate of prostate cancer in our studied population was very low; therefore, we do not recommend routine PSA screening in Saudi Arabia. This recommendation is in line with most international guidelines.
Acknowledgment.
The authors appreciate Ms. Suad Alsoghayer’s help with the statistics.
Footnotes
Disclosure. Authors have no conflict of interests, and the work was not supported or funded by any drug company.
- Received February 24, 2019.
- Accepted May 16, 2019.
- Copyright: © Saudi Medical Journal
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.