Abstract
Objectives: To evaluate the influence of periodontal therapy on glycosylated hemoglobin and fasting blood glucose and serum levels of interleukin (IL)-4, IL-6, IL-8, IL-10, and tumor necrosis factor-alpha (TNF-α) in chronic periodontitis (CP) patients with type-2 diabetes mellitus (T2DM) and in controls.
Methods: A total of 30 periodontal patients, 15 of which were systemically healthy (control group), and 15 were T2DM patients (test group) were included in this study. This prospective study was carried out at Istanbul University, Istanbul, Turkey between February 2011 and December 2013. Plaque index, gingival index, bleeding on probing, periodontal probing depth, and clinical attachment level were assessed and recorded at baseline, one, and 3 months after therapy. Serum samples were collected at the same time-points and analyzed using Luminex assay for the levels of IL-4, IL-6, IL-8, IL-10, and TNF-α. The change in the metabolic control was also monitored.
Results: All clinical parameters were significantly improved after the periodontal therapy in both groups (p<0.001). Glycosylated hemoglobin levels were decreased; however, the difference was not significant (p>0.05). Fasting blood glucose levels were decreased one month after therapy, and increased at 3 months. Patients with T2DM had significantly higher levels of circulating IL-8 at each time point, and TNF-α (p<0.05) at baseline. The IL-4 and IL-10 levels were decreased at one month after therapy (p>0.05).
Conclusion: Periodontal therapy has limited impact on the serum levels of IL-4, IL-6, IL-8, IL-10, and TNF-α. Metabolic control levels were not influenced by periodontal therapy.
Chronic periodontitis (CP) is an infectious disease resulting in inflammation in periodontal tissues, progressive attachment, and bone loss. Chronic periodontitis is the most common type of periodontitis, and its prevalence and severity increases with age.1 Microbial dental plaque is the main etiological agent; however, progression from gingivitis to periodontitis is associated with host response and immunity. Presence of systemic disease such as diabetes, stress, and genetic factors are among the factors related to host response.2 Bone loss occurs with the influence of local factors, which are expressed from inflammatory mediators. Interleukin (IL)-1β, tumor necrosis factor-alpha (TNF-α), IL-6 are the cytokines favoring bone loss around the teeth. It has been reported that patients with chronic periodontitis present with increased systemic inflammation and raised levels of various inflammatory markers compared with healthy controls.3 The local tissue produces inflammatory cytokines, as well as elevates their systemic circulating levels. Type-2 diabetes mellitus (T2DM) is a multifactorial metabolic disorder characterized by chronic hyperglycemia with disturbances of carbohydrate, fat, and protein metabolism. Defects in insulin secretion (b-cell dysfunction), insulin action (insulin resistance), or both cause T2DM.4 Type-2 diabetes is regarded as a low-grade inflammatory disease because some inflammatory cytokines are involved in the mechanism. Functions of specific immune system cells are impaired in patients with DM. Diabetes mellitus causes dysfunction in the adhesion, chemotaxis, and phagocytosis capacity of neutrophils. As a result, they cannot kill periodontopathogens, and cannot destroy their toxins resulting in the destruction of periodontal tissues,5-7 which may explain in part the increased incidence of periodontitis among diabetic patients. The glycosylated hemoglobin (HbA1c) levels reflect the glycemic level over the previous one to 3 months. Whether periodontal therapy reduces HbA1C levels in periodontitis patients remains controversial.8-14 In patients with periodontitis, diabetes is associated with elevated levels of several cytokines and other mediators in serum, saliva, and gingival crevicular fluid (GCF). It was reported that monocytes in peripheral blood of patients with DM produce higher amounts of TNF-α when encountered with Porphyromonas gingivalis.15
The TNF-α, which is a pro-inflammatory cytokine was first reported by Hotamisligil et al16 to cause insulin-resistance. The TNF-α produced by the adipose tissues acts as a risk factor for periodontal disease; likewise, TNF-α produced due to periodontal inflammation may be an additional risk factor influencing insulin sensitivity.17 The TNF-α, IL-6, resistin, and other pro-, and anti-inflammatory cytokines activate intracellular pathways, which causes the development of insulin resistance and T2DM.18 Interleukin-4 reduces secretion of IL-1, IL-6, and TNF-α from monocytes.19 It was reported by a series of studies that patients with diabetes have a lower concentration of GCF IL-4 compared with healthy subjects.20-22 It was indicated that periodontal therapy reduces serum IL-6 concentration significantly in patients with CP.23 Interleukin-8 is a chemo attractive proinflammatory cytokine that affects neutrophil migrations in thetissue and circulation. Interleukin-1β and TNF-α has a role on IL-8 production.24 Substantial data has been accumulated on metabolic measures and serum levels of cytokines on T2DM patients with periodontitis. A systematic review has shown that non-surgical periodontal treatment results in a mean reduction in HbA1C of 0.36% (95% confidence interval [CI]: 0.19-0.54) at 3 months.25 However, the results from different reports are not consistent to demonstrate the potential effects of non-surgical periodontal therapy on Hba1C and specific cytokines in T2DM patients with CP.9-14
We hypothesized that non-surgical periodontal therapy will reduce the levels of proinflammatory cytokines, HbA1c, and fasting blood glucose (FBG) levels in T2ßDM patients with CP, and this group of patients can benefit from periodontal therapy, as well as non-diabetics. Thus, the aim of this study was to evaluate the influence of periodontal therapy on HbA1C and FBG and serum levels of IL-4, IL-6, IL-8, IL-10, and TNF-α in CP patients with T2DM and in controls.
Methods
Study population, design, and clinical procedures
A total of 30 subjects with chronic periodontitis were recruited into the present study between February 2011 and December 2013. Fifteen subjects with T2DM constituted the test group, and 15 systemically healthy subjects were included in the control group. Patients with T2DM were referred from the Department of Metabolic Disease, School of Medicine, Istanbul University, Istanbul, Turkey after their routine outpatient visits. Patients with T2DM were being monitored by the same physician at least 2 years prior to study, and the diagnosis of T2DM was made using the criteria of the American Diabetes Association.4 All patients were receiving prescribed oral hypoglycemic agents and/or insulin for the treatment of diabetes. Patients were excluded if they have had any systemic disease other than T2DM, previous periodontal treatment, received antibiotics in the last 6 months, were pregnant or lactating, or allergic to the local anesthetics. Systemically healthy patients were selected among the patient group referred to the Periodontology Department, Istanbul University, Faculty of Dentistry. These patients had not received periodontal treatment before, and not used antibiotics within the last 6 months. The FBG and HbA1C tests were obtained from each subjects before baseline in the control group to verify that they were diabetes-free. All patients had a clinical diagnosis of chronic periodontitis,26 with at least 4 teeth in each jaw with a probing depth (PD) ≥5 mm, clinical attachment level (CAL) ≥4 mm, at least 2 single-rooted teeth with a PD of 6-9 mm, and bleeding on probing (BoP). Smoking habit was restricted to mostly 10 cigarettes/day for each group. The body mass index (BMI) scores were measured and recorded at baseline. Periodontal measurements included plaque index (PI; Silness-Löe),27 gingival index (GI; Löe-Silness),28 PD, BoP (presence or absence of bleeding in the 20 seconds after probing), and CAL. All measurements were repeated at one and 3 months after therapy. Measurements were obtained at 6 sites around all teeth, excluding the third molars, using a periodontal probe by the same calibrated investigator blinded to the systemic situation of the patients. An intraclass correlation coefficient of 0.85 for PD measurements indicated that the intra-examiner reliability was high. Sample size and power were calculated prior to study. Scaling and Root Planing (SRP) were performed in 2 sequential visits in 7 days using the combination of hand instruments and ultrasonic devices (Hu-Friedy, Dentsply, IL, USA) by another investigator who was also blinded to patient distribution. Patient distribution to the investigators for clinical measurements and periodontal therapy were made by a third investigator. Patients in both groups received no adjunctive therapy. All subjects were followed-up monthly, oral hygiene instruction was repeated, and supragingival scaling performed when necessary during study period. The present study was approved by the Ethical Committee of Istanbul University, School of Medicine. All volunteers were informed of the aims and methods of this study and gave their written consent to participate before enrollment into the study. The study was carried out in accordance with the Helsinki Declaration.
Serum sampling
Five milliliters of blood were obtained from the antecubital vein by venipuncture and serum was separated by cool centrifugation at 3,000 rate/minute (rpm) for 10 minutes at +4°C. Separated serum samples were collected in Eppendorf tubes and immediately transported to -80°C for freezing and storage.
Cytokine analyses
The plasma concentrations of IL-4, IL6, IL-8, IL-10, and TNF-α were analyzed using the multiplex bead technique device (AtheNa Multi-Lyte ANA Test System, Zeus Scientific Inc, Branchburg, NJ, USA) using commercially available Milliplex high-sensitivity kits (Millipore Corporation, City/State USA by Luminex) according to the instructions of the manufacturer. The results were calculated using a specific software (Milliplex Analyst Software, Millipore, EMD Millipore, Billerica, MA, USA)
Fasting blood glucose, and HbA1C measurements
Venous blood samples were obtained from the study population at baseline, and one, and 3 months after therapy from the test group. Blood was centrifuged for 10 minutes at 4,000 rpm. The FBG (mg/dl) and HbA1C (%) were determined in plasma samples. The duration of T2DM, body weight, and height of each patient with DM were also recorded. Plasma analyses were performed at the Department of Clinical Biochemistry, School of Medicine, Istanbul University, Istanbul, Turkey.
Data analysis
Data was analyzed using a statistical software (Pass 2008/NCSS 2007, NCSS, Kaysville, UT, USA). For PD and CAL, measured in millimeters, metabolic control, and BMI, a mean value of each individual was first obtained, and thereafter a mean value was calculated for the group. For analysis of inflammatory biomarkers, a value of each individual was first obtained, and then a mean value was calculated for the group. Kolmogorov-Smirnov test was used to test the distribution of the data. The changes in the clinical and metabolic data were presented as mean and SD, and the changes in inflammatory biomarker data were presented also as mean values and SD. The ANOVA and Friedman tests were used to detect differences within groups over time for repeated measures of clinical parameters and serum levels of cytokines. The differences in clinical, immunological, and metabolic control data between groups were analyzed using parametric methods (Student t-test) if the data distributed normally. The differences between groups were analyzed using a non-parametric method (Mann Whitney U test) if the data did not distribute normally. P<0.05 was accepted as statistical significancy. Bonferroni correction was used for paired intra-group comparisons, and p=0.016 was accepted as statistically significant. A p<0.05 was accepted as significant for inter-group comparisons. Prior to the initiation of the study, sample size, and power were calculated. Type-1 error was assumed at 0.05, type-2 error was assumed at 0.02, and power was assumed at 80% predicting a 30% reduction between baseline and 3 months.
Results
Demographic and clinical findings
The demographics of study participants are shown in Table 1. A total of 30 subjects with periodontitis were recruited into the present study. Fifteen patients with T2DM and chronic periodontitis, and 15 systemically healthy patients with chronic periodontitis were monitored. The sample was composed of 13 men and 17 women; mean age was 45.8 years with the range of 33-58 years, while mean diabetes duration was 6.93±3.65 years. Initially, both groups had a similar number of sites that were affected from periodontitis severely.
Demographic data and body mass index (BMI) of the study population.
The SRP led to improvements in all clinical periodontal parameters of both groups at one and 3 months compared with baseline (p<0.05). Table 2 shows the change in periodontal parameters at baseline, one, and 3 month follow-up examinations. The mean PI was significantly decreased in both groups (p<0.05); however, there was no significant difference between groups at baseline (p=0.117), one (p=0.273), and 3 months (p=0.055) after therapy concluding good plaque control in both patient groups. Parallel to PI scores, GI was decreased significantly after therapy in both groups at one and 3 months compared with baseline. Inter-group comparisons revealed that there was no significant difference at baseline in GI scores however, the test group had significantly higher GI scores compared with controls at one (p=0.011) and 3 months after therapy (p=0.0001).
Mean ± standard deviation for plaque index (PI), gingival index (GI), bleeding on probing (BoP), periodontal probing depth (PPD), and clinical attachment level (CAL) of study population at baseline, one, and 3 months after therapy.
The SRP was successful on PD and CAL measurements in both groups with no significant difference between groups at any observational period. The treatment resulted in significant improvement at one and 3 months compared with baseline, and there was no significant difference between one and 3 months in both groups (p=0.0001) for both groups for either comparison) in PD measurements. Similar to mean PD scores, there was significant gain in CAL in both groups with no significant difference between the test and control group. Active inflammation in the periodontal pockets, as assessed by BoP measurements, reduced significantly after therapy compared with baseline in the test and control groups. There was no significant difference between the groups at baseline and after therapy (Table 2).
Fasting blood glucose and HbA1C levels
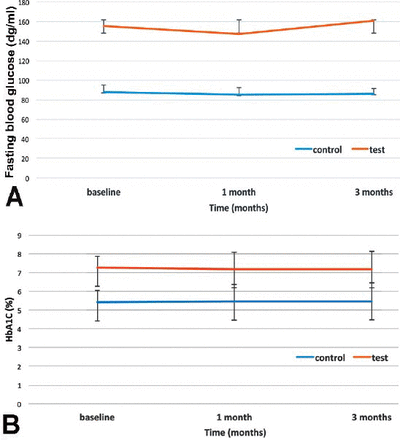
Periodontal therapy did not lead to a significant change in patients with or without diabetes in the levels of FBG (p>0.05). The test group had significantly higher levels of FBG at baseline and one and 3 months after therapy than the control group (Figure 1A). The HbA1C levels of the subjects with T2DM were not improved by the therapy (7.27±0.615 at baseline, and 7.18±0.97 at 3 months) (p>0.05). Also, HbA1C levels of the control group remained steady throughout the study period (5.42±0.283 at baseline, and 5.48±0.297 at 3 months) (p>0.05). There were statistically significant differences between the test and control groups at baseline (p=0.0001), one, and 3 months (p=0.0001) after therapy (Figure 1B).
Different monitoring periods for fasting blood glucose (FPG) and glycosylated hemoglobin levels (HbA1C): A) Test group had significantly higher FPG levels compared to the control group (p<0.001). There was no significant change within groups from baseline to one and 3 months (p>0.05); and B) Test group had significantly higher HbA1C levels compared to the control group (p<0.001). There was no significant change within groups from baseline to one and 3 months (p>0.05).
Cytokine levels in serum
The IL-4, IL-6, IL-8, IL-10, and TNF-α were analyzed in the present study. In the control group, most cytokines analyzed in the present study were influenced by the therapy at one month compared with baseline and reduced however, this change did not reach significance in either cytokine levels in serum. Serum levels of TNF-α followed a different pattern showing a slight increase at one month, and a more prominent increase at 3 months after therapy compared with baseline (p>0.016) (Table 3).
Mean ± standard deviation of serum inflammatory markers at baseline, one, and 3 months after therapy.
In the test group, IL-4, IL-8, and TNF-α levels increased at one month after therapy with no significant difference (p>0.05). The IL-6 and IL-10 were the cytokines that showed reduction after therapy, although the change was not significant (p>0.05). Considering inter-group analyses, IL-8, and TNF-α were significantly lower at baseline in the control group than test group (p<0.01 and p<0.05). In addition, serum levels of IL-4, IL-8, and IL-10 were lower in the control patients compared to test patients at one month after therapy (p<0.01, p<0.01, and p<0.05). The IL-4 and IL-8 levels remained at significantly lower levels at 3 month in the control group compared with the test group (p<0.05 and p<0.01) (Table 3).
Discussion
The association between periodontitis and diabetes have been studied extensively. The effect of non-surgical treatment on diabetic patients has been demonstrated. In our study, we evaluated the effects on serum parameters, and our results indicated that the non-surgical periodontal therapy did not significantly change FBG and HbA1C levels after 3 months in T2DM patients with periodontitis. Likewise, serum levels of IL4, IL-6, and IL-8 were not influenced significantly by the therapy applied in the diabetic and non-diabetic periodontitis patients after one and 3 months. Although the difference was not significant, serum TNF-α levels in diabetic and non-diabetic patients were increased after 3 months, and IL-10 levels were decreased in non-diabetics after one month.
The TNF-α, which has been reported to play a key role in the pathogenesis of T2DM,29 and to be correlated with insulin resistance30,31 increased after the therapy in systemically healthy patients and patients with DM in this study. This finding agrees with other studies that reported an increase in circulating TNF-α levels after therapy.32-35 In contrast, some studies reported decreased TNF-α levels after therapy in association with local antibiotic delivery,36 or not.37,38 Duration and type of diabetes, severity of periodontal situation, and the use of adjunctive therapies may account for these differences among studies.
Another main finding of the present study was that circulating IL-8 levels were significantly higher in DM patients than systemically healthy individuals at baseline and after therapy, which indicates that this cytokine tended to increase throughout the study. The IL-8 has a role to activate circulating or tissue-resident neutrophils during infection and inflammation.22 This finding is consistent with the findings of Lalla et al,34 and O’Connel et al.35
Our results were inconsistent with other studies reporting lower levels in GCF of DM patients than systemically healthy patients,39 and non-significant differences between DM and systemically healthy patients.40
The IL-4 is an anti-inflammatory cytokine which is expressed by T cells, mast cells, and basophils, and has a role in decreasing the expression of IL-1, TNF-α, and IL-6 from monocytes.16 The detection of IL-4 was below 25% with the lowest detection ratio among the cytokines investigated in the present study. The IL-4 was detected in the samples of a total of 10 patients; only 2 of which were systemically healthy. As a result, we could not compare IL-4 levels with other studies.
The IL-10 is another cytokine investigated in the present study. The mean levels of circulating IL-10 decreased after the therapy. However, the difference was significant only in systemically healthy controls. Periodontal therapy had a limited impact on the expression of IL-10 in diabetic patients, and this finding is consistent with other studies reporting non-significant decrease after therapy at 3 months,35,38 and inconsistent with the findings of Lalla et al,34 who reported an increase after therapy, while the difference was not significant.
Very recently, in a multicenter study, it was reported that non-surgical periodontal treatment for participants with DM and chronic periodontitis did not demonstrate a benefit to measures of glycemic control (n=257),25 contrary to the findings of Sun et al,41 which reported a non-statistically significant HbA1c reduction of 0.36% in the treatment compared with the control group after 4 months. Sun et al41 divided a total of 50 impaired glucose tolerance (IGT) and 106 T2DM patients with periodontitis into 3 groups: IGT group (50 patients), T2DM without macrovascular disease group (DM1 group, 58 patients), and T2DM with macrovascular disease group (DM2 group, 48 patients). These patients were divided into subgroups whether they performed periodontal intervention. All clinical parameters improved 3 months after periodontal intervention in accordance with our findings. Contrary to our results, the serum HbA1c levels were also improved after 3 months of periodontal intervention (p<0.01). In DM2 group, IL-6, and TNF-α did not change after therapy consistent with our findings. In our study, there was a decrease in HbA1C levels, although this decrease did not reach significancy. This is in line with past studies.11,38,42,43 Conversely, there is substantial data from a number of studies supporting the positive role of periodontal therapy on metabolic control.12,35,36,44 In some of these studies, adjunctive local antibiotics were applied following mechanical intervention. In this respect, this effect of periodontal therapy on metabolic control may in some part be because of antibiotics,45 and not solely on mechanical debridement. Adjunctive use of systemic antibiotics to enhance the results of SRP on the periodontal situation, and the effects of mechanical debridement over glycemic control were also assessed by a number of studies.46,47 Tsalikis et al46 reported no additional effect of doxycycline over SRP alone for clinical periodontal parameters, and values of HbA1c in T2DM patients after 3 and 6 months. On the contrary, Miranda et al47 reported that adjunctive use of metronidazole plus amoxicillin improved the clinical and microbiological outcomes of SRP in T2DM patients with chronic periodontitis. The diabetic patients in this study were within the limits of obesity (mean BMI; 32.69±4.98 kg/m2) and the increased adipose tissue may induce the production of some cytokines (namely, IL-6, TNF-α), and thereby worsen the response to periodontal therapy preventing improvement in metabolic control.
When the periodontal clinical parameters of the present study were evaluated, it was observed that the results were similar at baseline, and improved significantly after therapy with no significant difference between the groups. The GI and BoP values were improved significantly in both groups after the therapy. However, periodontal inflammation as assessed by GI and BoP were significantly decreased to lower levels in the control group compared with the T2DM group after the therapy. The clinical findings of this study are consistent with other studies with similar treatment protocols.42,48 There is increasing evidence that diabetes impairs resolution of inflammation and repairs after periodontal therapy,49 which leads to poorer response in periodontal tissues to treatment. This is even more evident in unregulated diabetic patients.50 In the present study, patients in the test group were within the limits of regulation of diabetes (mean HbA1C; ≤7.5%) and as a result, diabetic patients responded very well to treatment.
It has been shown that smoking interferes with healing after non-surgical periodontal therapy and smokers show less reduction in the counts of periodontal pathogens compared with non-smokers.51 In the current study, there were no subjects who smoked more than 10/day. The ratio of smokers to non-smokers (3/15 in the test group, versus 4/15 in the control group) were also comparable. In this respect, both groups were affected from the detrimental effects of smoking similarly.
The limitations of the study
This sample size of the study was limited. A positive control group with no periodontal treatment would be desirable to observe the effects of periodontal therapy on metabolic parameters and serum cytokine levels. Further studies are necessary to answer the question of how regulated and unregulated diabetic patients respond to periodontal treatment in terms of serum cytokine levels and periodontal findings. According to our results, no difference was detected after periodontal therapy in serum cytokine levels and metabolic measures. One reason might also be that more obvious inflammation was necessary to observe the difference. A study including aggressive periodontitis patients or periodontitis patients with clearer inflammation might show some difference after periodontal therapy.
The most prominent data of the study was that circulatory IL-8 and TNF-α were significantly higher in diabetic patients at baseline as a possible evidence of increased inflammatory response in diabetics against local infection. However, the increase could also be related to the diabetic situation of the patients. Furthermore, HBA1C was decreased but this was not significant. Secondly, we did not observe any difference in clinical periodontal parameters after periodontal therapy between the groups.
In conclusion, our results showed improved periodontal parameters concluding that T2DM patients can benefit from periodontal therapy, as well as non-diabetic patients with periodontitis. However, periodontal therapy did not provide an impact on the levels of metabolic control, or serum inflammatory markers in patients with T2DM and periodontitis.
Acknowledgment
The authors gratefully acknowledge Dr. Sami Fatayer for the translation of the abstract and title page into Arabic.
Footnotes
Disclosure. This study was funded by the Research Fund of Istanbul University (Project no: 3296), Istanbul, Turkey.
- Received October 28, 2014.
- Accepted February 2, 2015.
- Copyright: © Saudi Medical Journal
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.