Abstract
Objectives: To evaluate the role of applying a limited panel of immunohistochemical stains on the cellblock preparation from samples obtained by endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) in the aim of differentiating solid pseudopapillary neoplasms (SPNs) from neuroendocrine tumors (NETs).
Methods: We retrospectively retrieved all the EUS-FNAs of the pancreas that have a diagnosis of NET or SPN that were performed at 2 tertiary care hospitals in Riyadh, Kingdom of Saudi Arabia from May 2004 to December 2014. Diff-Quik, Papanicolaou, and Immunohistochemistry stains on cellblock preparations were performed.
Results: Twenty cases were available (16 pancreatic neuroendocrine tumors (pNETs) and 4 SPNs). The pNETs were immunoreactive for synaptophysin, chromogranin A and CD56 while E-cadherin was diffusely to focally cytoplasmic positive. β-catenin was negative or showed focal cytoplasmic immunoreactivity. In comparison, SPNs were positive for vimentin, CD10, CD-56, focally positive for progesterone receptors and synaptophysin, and revealed nuclear immunostaining for β-catenin. They were negative for chromogranin A and E-cadherin.
Conclusion: Based on EUS-FNA samples, nuclear immunoreactivity for β-catenin with loss of membranous immunostaining for E-Cadherin can potentially facilitate differentiating SPNs from pNETs.
The vast majority of solid pancreatic tumors are ductal adenocarcinomas, while the remainder includes neuroendocrine, acinar cell tumors and solid pseudopapillary tumors. Tumors other than ductal adenocarcinoma may be more amenable to therapeutic intervention and thus histological diagnosis is essential.1 Pancreatic neuroendocrine tumors (pNETs) are relatively uncommon and account for 1-2% of all pancreatic neoplasms,2 while solid pseudopapillary tumors (SPTs) are another uncommon low-grade malignant neoplasm accounting for 1% of all exocrine pancreatic tumors.3 Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) has been widely used to sample pancreatic lesions and has a sensitivity of 89% (confidence interval [CI]: 88-90%) and specificity of 96% (CI: 95-97%).4 It still remains 1 to 14% (pooled mean 5%) of EUS-FNA results that are reported as “atypical” in studies that lack a more specific diagnosis.5 This atypia could be related to reactive changes, preparation artefacts, well-differentiated duct carcinoma, as well as uncommon malignant neoplasms.5 The aim of the present study is to evaluate the role of a limited panel of immunohistochemical stains on cellblock preparations obtained from EUS-FNA to differentiate between pNETs and solid pseudopapillary neoplasms (SPNs).
Methods
After an Internal Review Board approval was obtained in the participating institution, we retrieved all EUS-FNAs of solid pancreatic lesions that were performed at King Khalid University Hospital, King Saud University, and King Fahad Medical City in Riyadh, Kingdom of Saudi Arabia from May 2004 to December 2014. We included cases where a diagnosis of a pNET or SPN was made. Cases without adequate cytomorphologic material/features or confirmative surgical samples were excluded. All cases had an EUS performed using a linear echoendoscope and FNA was obtained with either a 21 or 25-gauge needle based on the discretion of the endoscopist. Smears were made onsite in the endoscopy suite using the Rapid On Site Evaluation (ROSE) by our cytotechnologist in order to assess sample adequacy. The aspirated material was expelled on glass slides by the operating endoscopist and smeared by a cytotechnologist. Two to 4 slides were prepared from each pass, and clotted material was preserved for cellblock. Air-dried (for Diff quick staining) and fixed smears (fixed immediately in 95% ethyl alcohol for subsequent Papanicolaou staining) were prepared in an almost equal ratio with more emphasis on fixed smears. Pass number was marked on each slide and the site of collection of each pass was noted. One or 2 representatives air-dried smears from each pass were immediately stained with rapid modified Romanowsky (Diff Quick stain, Shandon Corp, Aukland, New Zealand) and examined under a microscope in order to assess specimen adequacy, give preliminary diagnostic interpretation if necessary, and to suggest additional studies if indicated. The Roswell Park Memorial Institute (RPMI) cell preservative solution was used as a cell collection/preservation and transport medium for cellblock and subsequent ThinPrep slides preparation. The material collected for cellblock was grossly examined before the end of the procedure and if the collected material was not sufficient to make a cellblock, additional passes were requested and dedicated to cellblock only.
Preparation of ThinPrep slides
The RPMI Needle wash was centrifuged immediately and an aliquot was separated for ThinPrep processing and processed (ThinPrep® 2000 machine, Marborough, MA, USA) according to the manufacturer’s instructions of ThinPrep processing manual.
Preparation of cellblock
The remaining sediment including any clotted material was fixed immediately in a cellblock fixative (10% Alcohol formalin), centrifuged and the material transferred into a histology embedding cassette, and processed for routine histologic examination using standard techniques. Fourteen out of 20 patients underwent surgical resection. Histologic sections (approximately 5 µm) were cut from formalin-fixed and paraffin-embedded tissue blocks and stained with hematoxylin and eosin. Appropriate immunohistochemical (IHC) studies were performed on cellblocks and surgical specimens. For this purpose, approximately 5 µm sections were cut, deparaffinised and mounted on pre-coated slides.
The following antibodies were used for immunocytochemistry (ICC) assessment: synaptophysin, chromogranin A, CD56, progesterone, β-catenin, E-cadherin, CD10, and vimentin (Novocastra inc., Newcastle, UK). All included cases had a confirmative diagnosis either by cytomorphological and immunocytochemical findings or by subsequent histopathologic examination of the surgical excision specimens.
Results
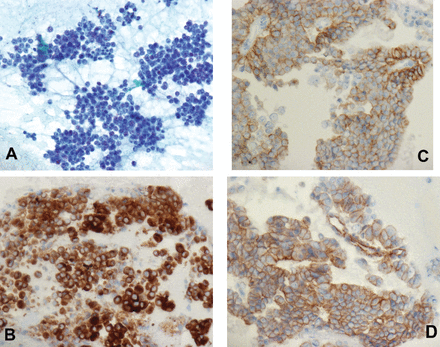
Sixteen patients (Males=9, Females=7; age range 31-87 year) with pNETs were diagnosed by EUS-FNA cytology. The FNA smears were highly cellular in all the cases. The aspirates revealed predominantly single cell population and often contained loosely cohesive groups and rosette-like formations. Cells were small to medium in size with a moderate amount of pale to eosinophilic cytoplasm and remarkably uniform, monotonous, small to medium-sized, round to oval, and frequently peripherally located (plasmacytoid appearance) nuclei with finely distributed, ‘‘salt-and-pepper’’ chromatin. Nucleoli were inconspicuous, or small. The background frequently was bloody. Mitotic figures and necrotic cell debris were noted rarely (Figure 1A). Most tumors were diagnosed as pNETs according to the cytomorphologic features and were further confirmed by positive immunostaining for neuroendocrine markers. In all cases the neuroendocrine markers including synaptophysin (Figure 1B), chromogranin A and CD56 were diffusely to focally positive. E-Cadherin was diffusely to focally positive with membranous distribution (Figure 1C) and β-catenin was negative or showed focal cytoplasmic positivity (Figure 1D).
Pancreatic endocrine neoplasm, highly cellular aspirate composed of uniform, discohesive cells and A) revealing the typical finely distributed “salt-and-pepper” chromatin, Pap Stain (50x), B) Immunohistochemistry stains performed on cellblock showed that tumor cells are positive for synaptophysin, IHC stain (40x), C) with membranous positivity for E-cadherin, IHC stain (40x), D) IHC staining for B-catenin revealed membranous/cytoplasmic distribution with clear absence of nuclear staining, IHC stain (40x). IHC - immunohistochemical
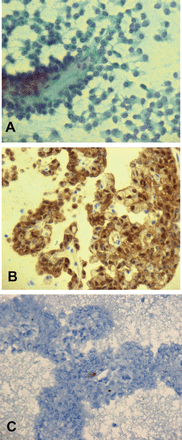
Few tumors were diagnosed as suspicious of pNETs; as immunostaining was not available because of the lack of sufficient cellblock material. However, the surgical resections confirmed the pNET diagnosis in all these cases. Four females were diagnosed as SPTs by EUS-FNA cytology. They were 19, 33, 37 and 44 years old. The smear and cellblock preparations were highly cellular with numerous papillary fronds containing thin fibrovascular cores with round or oval nuclei, small nucleoli and abundant cytoplasm (Figure 2A). There was no evidence of pleomorphism or mitotic activity. Foamy cells, blood and debris were present in the background. The cellblock specimens were immunoreactive for vimentin, CD-56, with focal positivity for progesterone receptors and synaptophysin. Nuclear immunostaining for β-Catenin (Figure 2B) was noticed. The tumor cells were non-immunoreactive for chromogranin A, and E-cadherin (Figure 2C). This diagnosis of SPT was confirmed by histopathologic examination of the surgical resection specimens on all these cases. Ki-67 was only available at one of the participating sites and the Ki-67 index was more than 85% in 25% of the cases at that site.
Solid pseudopapillary neoplasm, cellular smear with loose clusters as well as scattered well-formed papillary structures. A) The cells have delicate to clear cytoplasm and bland nuclei with fine chromatin, Pap stain (40x), B) Immunohistochemistry stains obtained on cellblock preparation showed that tumor cells are positive for B-catenin, nuclear pattern, IHC stain (40x), and C) Same cells are negative for E-cadherin, IHC stain (40x). IHC - immunohistochemical
Discussion
Pancreatic neuroendocrine tumors account for 1-2% of all pancreatic neoplasms with an incidence of 1-4 per 100,000,6,7 with most classified as low to intermediate grade neuroendocrine tumors (NETs) having a relatively “indolent” clinical course and only a minority are high-grade. In a meta-analysis, EUS detected pancreatic NETs with a sensitivity of 87.2% (95%CI; 82.2 to 91.2) and specificity of 98.0% (95%CI; 94.3 to 99.6).8
Solid pseudopapillary neoplasms of the pancreas are low-grade malignant neoplasms that account for approximately 1-2% of all pancreatic tumors. In a systematic review the majority of these tumors occur in females (87.8%) with a mean age of 28.5 years.9 These tumors are often relatively large at the time of presentation; however, in 85% of patients, the tumors are confined to the pancreas. Patients with SPNs have an excellent prognosis after complete surgical resection10 with a disease free survival of 96% while the recurrence rate was 4%.9 Of note, the rates of reporting these tumors increased seven fold since 2000.9
Although SPN’s have characteristic features on EUS including a well demarcated lesion without internal septation or main pancreatic ductal dilatation,11 the presence of dense rim calcifications can obscure examination of the internal content of these lesions thus relying on the cytological findings to make a diagnosis is critical.11 In a multinational, muti-center study over 15 years there were 34 patients diagnosed to have SPN’s.12 The addition of FNA to conventional imaging increased the diagnostic yield for SPNs from 23.5% for CT scans and 41.2% for EUS alone to 82.4% for EUS when FNA was added.12 The preoperative diagnosis of these tumors using EUS-FNA is, hence, important because of the different biologic behaviour and management, as SPN’s usually require only limited resection with an attempt to preserve the function of the pancreas.
NETs and SPNs share many cytological features.13 Aspirates from both tumors may yield moderate to very high numbers of cells. Both of these tumors also demonstrate single cells with low nuclear–to–cytoplasmic ratios. They may also demonstrate a plasmacytoid appearance and may show conspicuous but not prominent nucleoli.14 A recently described finding of SPNs that help discriminate these tumors from pNETs and acinar cell tumors is the presence of cercariform cells.15
Performing IHC studies on cellblock can help in distinguishing SPNs from pNETs as the management of both tumors differ significantly. However, SPNs can express some markers seen in pNETs including neuron-specific enolase, CD56, synaptophysin and occasionally chromogranin A.16-18 CD10 is expressed in SPNs but about 25% of pNETs also demonstrate focal immunoreactivity.19 β-catenin and E-cadherin immunostaining can help in differentiating SPN from pNET. β-catenin is a principal member of the E-cadherin/catenin complex. It has been described that the majority of cases of SPN’s show β-catenin gene mutations, leading to cytoplasmic and nuclear accumulations of β-catenin.17,18 SPNs also show complete loss of membranous and cytoplasmic expression of E-cadherin with nuclear localization. This combined immunoprofile of E-cadherin and β-catenin seems to be exclusive to SPNs.20 Progesterone and vimentin support the diagnosis of SPNs but they should not be used in isolation.18
In our study, we applied a panel of immunohistochemical staining that included synaptophysin, chromogranin A, CD56, progesterone, β-catenin, E-cadherin, CD10 and vimentin on cellblock preparation of three cases of pNETs and 3 cases of SPNs. We found that all the SPNs showed nuclear positivity for β-catenin, with membranous CD56 and CD10 and all were non-immunoreactive for E-cadherin.
Our results are similar to that reported by Notohara et al17 and Burford et al18 who suggested that the lack of membranous immunostaining with E-cadherin antibodies and positive nuclear staining with β-catenin support the diagnosis of SPNs with a specificity of 100%. Application of Ki-67 staining to cellblock material of NETs may have value in stratifying tumors into low and high-grade forms and has been included in the World Health Organization classification of pNETs21,22 and potentially a prognostic value.23,24 Potential pitfalls in the evaluation of solid pancreatic lesion by EUS-FNA include interpretation, sampling or misclassification errors25 and most misclassifications occur in cases of SPN’s.25 Therefore, it is recommended in some difficult cases to include these 2 entities as a final differential diagnosis. The current study, though limited by the low number of cases that were included, it has shown the importance of a limited panel of immunohistochemistry stains in differentiating pNETs and SPNs on EUS-FNA cytology specimens.
Clinical Practice Guidelines
Clinical Practice Guidelines must include a short abstract. There should be an Introduction section addressing the objective in producing the guideline, what the guideline is about and who will benefit from the guideline. It should describe the population, conditions, health care setting and clinical management/diagnostic test. Authors should adequately describe the methods used to collect and analyze evidence, recommendations and validation. If it is adapted, authors should include the source, how, and why it is adapted? The guidelines should include not more than 50 references, 2-4 illustrations/tables, and an algorithm.
Acknowledgment
The authors extend their sincere appreciation to the Deanship of Scientific Research, King Saud University, Riyadh, Kingdom of Saudi Arabia for its funding of this research.
Footnotes
Disclosure. This study was funded by the Deanship of Scientific Research at King Saud University, Riyadh, Kingdom of Saudi Arabia the Research Group Project number RGP-279
- Received December 8, 2015.
- Accepted May 25, 2016.
- Copyright: © Saudi Medical Journal
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.