Abstract
Transcutaneous spinal direct current stimulation (tsDCS) is a non-invasive method of stimulating spinal circuits that can modulate and induce changes in corticospinal excitability (CE) in incomplete spinal cord injury (SCI). A double-blinded sham controlled study of 2 male patients (A and B) with SCI was carried out. Patient A received sham and cathodal tsDCS, while Patient B received sham and anodal tsDCS. Four baselines were recorded prior to each arm of stimulation. Outcomes were then measured post each arm of stimulation; 10-meter walk test, modified ashworth scale, berg balance scale, manual muscle testing, and spinal cord independence measure-III. Transcranial magnetic stimulation, assessed motor evoked potentials. Cathodal tsDCS increased the scores in few of the outcome measures and decreased others. Anodal stimulation increased scores in all measures. Motor evoked potentials increased in post-cathode and deteriorated in post-anode. In conclusion, tsDCS modulated gait parameters, spasticity, and CE in incomplete SCI.
- spinal cord injury
- transcutaneous spinal direct current stimulation
- transcranial magnetic stimulation
- neuroplasticity
- corticospinal excitability
Based on an evolving body of evidence, neuroplasticity and sensorimotor remapping are believed to be mechanisms involved in functional recovery after neurological injury.1 Earlier studies focused on the role of repetitive training in facilitating neuroplastic changes in patients with spinal cord injuries (SCI), and they highlighted that intensive task-specific training protocols showed no superior effect on locomotion when compared to conventional physiotherapy.2 These findings led to the emergence of combination techniques where non-invasive brain stimulation is used with task-specific training to enhance neuroplastic changes in the central nervous system.3 Stimulation of the spinal cord, known as transcutaneous spinal direct current stimulation (tsDCS), is a non-invasive neuromodulatory technique that induces spinal segmental and higher cortical changes through neurotransmitter-dependent processes.4 Previous studies have revealed the effect of spinal modulation on the spinal segmental level as well as on the ascending and descending neural pathways in humans.5 In this study, the differential effects of tsDCS were investigated in 2 patients with chronic incomplete SCI (American Spinal Injury Association-C or ASIA-C). Functional measures such as walking speed, muscle strength, balance, and spasticity and neurophysiological parameters were assessed pre- and post-tsDCS.
Case Report
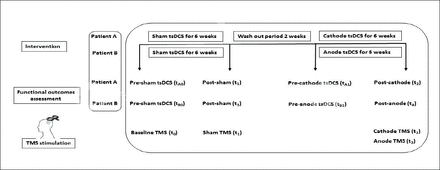
Two male patients, A and B, aged 22 and 24 years, respectively, with incomplete SCI type ASIA-C at spinal levels T10-T11 with a chronicity of 2 years were studied. Informed consents were signed prior to data collection, and the experiment was approved by the Institutional Review Board (IRB) at Imam Abdulrahman Bin Faisal University. This double-blind, sham-controlled study consisted of 2 arms for each participant. The first arm for patient A included sham tsDCS and the second included cathodal tsDCS. The first arm for patient B included sham tsDCS, while the second included anodal tsDCS. Each arm consisted of 30 sessions, with 5 sessions per week for 6 weeks with a washout period of 2 weeks between each arm of the study. Four baselines were measured prior to each arm of stimulation (Figure 1). Transcutaneous spinal direct current stimulation was administered by an independent assessor to ensure blindness.
Timeline summary of both patients’ clinical examinations, therapeutic interventions and follow-up. tsDCS: transcutaneous spinal direct current stimulation; (tA0): prior to sham tsDCS; (tA1): prior to cathode tsDCS; (t1): post-sham and (t2): post-cathode for patient A). (tB0): prior to sham tsDCS; (tB1): prior to anode tsDCS; (t3): post-sham and (t4): post-anode for patient B). TMS: transcranial magnetic stimulation; TMS (t0): baseline stimulation; TMS (t1): post-sham; TMS (t2); post-cathode and TMS (t3): post-anode.
Therapeutic interventions
Transcutaneous spinal direct current stimulation was administered online using a Magstim current stimulator (The Magstim Co., Whitland, UK) with saline soaked electrodes 5 × 7 cm. The active electrode was placed over the spinous process T10-T11 and the reference electrode was placed horizontally over the left deltoid muscle.6 Each session included 20 minutes of stimulation with a set intensity of 2.5 mA, resulting in a current density and total charge density of 0.07 mA/cm2 and 85.6 C/cm2. Sham tsDCS was ramped up only during the first 30 seconds of the session to ensure the procedure remained blind.4 Electrode placement was similar in all 3 arms of the experiment.
Intervention-Robot-assisted gait training
Both patients participated in a robot-assisted gait training program, while tsDCS was administered simultaneously. Gait training was performed on a Lokomat (Hocoma AG, Voletswil, Switzerland), which partially supports body weight using straps tied around the patient’s trunk. This system includes a treadmill, a body support harness, 2 light-weighted robotic arms connected to the legs, and a monitoring system for gait parameters. Functional gait training was facilitated using reciprocal walking movements of the lower extremities at the fastest speed that the patients could tolerate. The speed was then gradually increased by 0.1 km/hour every 10 min and lowered by 0.1 km/hour in case of poor foot contact or increased spasticity. To ensure good stance phase kinematics, the level of body weight support was adjusted to the minimum tolerated by the patient.
Outcome measures
Outcomes were measured at baseline twice: prior to sham tsDCS (tA0) and prior to cathode tsDCS (tA1) for patient A. Similarly, outcomes for patient B were also measured twice: prior to sham tsDCS (tB0) and prior to anode tsDCS (tB1). Outcomes were also measured immediately following each arm of tsDCS: post-sham (t1) and post-cathode (t2) for patient A and post-sham (t3) and post-anode (t4) for patient B. The primary outcome measure for functional gait changes was the 10-meter walk test (10MWT) which measures walking speed. Secondary outcome measures included the berg balance scale (BBS), modified ashworth scale (MAS), manual muscle testing (MMT), and the spinal cord independence measure-III (SCIM-III). The BBS measures balance during functional activities, MAS measures changes in spasticity, and MMT measures changes in muscle strength of hip flexion, hip extension, hip abduction, hip adduction, hip internal rotation, hip external rotation, knee flexion, knee extension, dorsiflexion, and plantar flexion with a cumulative score of 50. Finally, the SCIM-III determines patient functional ability. No formal statistical tests were used, as each outcome was represented by a single score.6 All post-training scores were compared to baseline scores for all outcome measures. For patient A, comparisons were made between baseline and sham tsDCS (t1-tA0 comparison) and between a second baseline and cathode tsDCS (t2-tA1 comparison). For patient B, comparisons were made between baseline and sham tsDCS (t3-tB0 comparison) and a second baseline and anode tsDCS (t4-tB1 comparison).
Neurophysiological parameters
To evaluate the integrity of the corticospinal tract excitability (CE), transcranial magnetic stimulation (TMS) was used to elicit bilateral triceps surae motor evoked potentials (MEPs). Motor evoked potentials amplitudes were measured at baseline (t0) for both patients A and B, post-sham (t1) for patient A and patient B, post-cathode (t2) for patient A, and post-anode (t3) for patient B. Motor evoked potentials recruitment was recorded at rest using a pair of Ag-AgCl surface electrodes (diameter =10 mm) placed bilaterally over the belly of the soleus muscles. A total of 5 MEPs were collected at ~10-s intervals with each TMS intensity ranging from 80% to 130% of the resting motor threshold (RMT). Resting motor threshold was measured at baseline (pre-training). Motor evoked potentials measurements were then averaged for each time point, and the MEP size was measured based on peak-to-peak amplitude for each stimulus intensity. Transcranial magnetic stimulation was delivered using a high-powered Magstim 2002 stimulator (Magstim Co., UK) fitted with a figure-of-8 coil with an external diameter of 70 mm placed over the motor cortex. Stimulation intensity (%RMT) was determined at rest based on the lowest stimulator intensity that is capable of evoking MEPs higher than 50 µV at peak-to-peak amplitude in at least 50% of 10 trials. Both patients completed all arms of the experiment without any reported complications.
Patient A
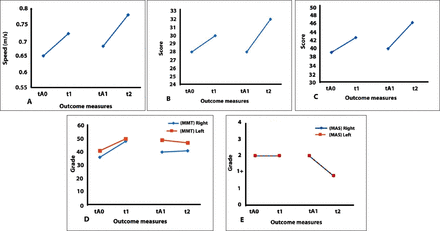
Speed on the 10MWT increased by 0.07 m/s following sham stimulation (t1-tA0 comparison) and showed a further increase of 0.06 m/s following cathodal stimulation (t2-tA1 comparison). The scores on the BBS increased by 4 points following sham stimulation (t1-tA0 comparison) and further increased by 4 points following cathodal stimulation (t2-tA1 comparison). Manual muscle testing of the right lower extremity muscle groups increased by 12 points following sham tsDCS (t1-tA0 comparison), but decreased by 7 points following cathodal tsDCS (t2-tA1 comparison). The left lower extremity showed an increase in 9 points on MMT scores (t1-tA0 comparison) and then decreased by 3 points following the second arm (t2-tA1 comparison). SCIM-III showed an increase of 2 points following each of the two arms of stimulation t1- tA0 and t2-tA1 comparisons. Finally, sham tsDCS showed no changes in MAS (t1-tA0 comparison), while cathodal tsDCS showed a decrease of one point (t2-tB1 comparison) for both lower extremities (Figure 2).
Graphical representation of outcome measures of patient A. A) 10 minute walk test (10MWT), B) spinal cord independence measure-III C) berg balance scal points, D) manual muscle test, and E) modified ashworth scale. tA0: first baseline prior to sham stimulation, t1: post-sham stimulation, tA1: second baseline prior to cathodal stimulation, t2: post-cathodal stimulation
Patient B
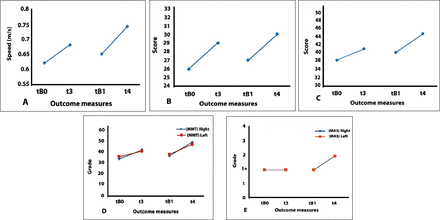
Walking speed, represented by the 10MWT result, increased by 0.06 m/s following sham stimulation (t3-tB0 comparison) and showed a further increase of 0.06 m/s following anodal stimulation (t4-tB1 comparison). BBS showed a continuous increase of 3 and 4 points following sham (t3-tB0 comparison) and anodal stimulations (t4-tB1 comparison), respectively. MAS showed no changes after sham tsDCS (t3-tB0 comparison), but the scores of spasticity were increased by 1 point for both the left and right lower extremities following the second arm of stimulation (t4-tB1 comparison). MMT of the right lower extremity muscle groups increased by 8 points following sham tsDCS (t3-tB0 comparison) and further increased by 7 points following anodal tsDCS (t4-tB1 comparison). The left lower extremity muscle groups showed a 5-point increase following sham (t3-tB2 comparison), and increased by 6 points following anode stimulation (t4-tB1 comparison). Spinal cord independence measure-III showed a 3-point increase following sham stimulation (t3-tB0 comparison), and scores increased by 1 more point following anodal stimulation (t4-tB1 comparison) (Figure 3).
Graphical representation of outcome measures of patient B. A) 10 minute walk test (10MWT), B) spinal cord indepence measure-III, C) berg balance scale, D) manual muscle test, and E) modified ashworth scale. tB0: first baseline prior to sham stimulation, t3: post-sham stimulation, tB1: second baseline prior to anodal stimulation, t4: post-anodal stimulation
Transcranial magnetic stimulation and MEP amplitudes
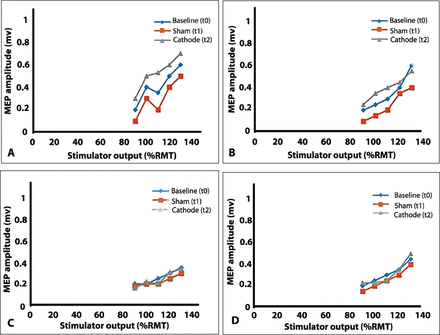
Patient A with an incremental increase of 10% of stimulus intensity (%RMT), the MEP amplitudes showed a gradual increase of 5 points on average (t0). Similarly, the increase in %RMT consistently increased MEP amplitudes post sham tsDCS (t1) and post cathode tsDCS (t2). These results were observed following both the right- and left-brain stimulation for Patient A.
Patient B, the increase in %RMT led to a parallel increase in MEP amplitudes at baseline (t0) and post sham (t1). Post anodal stimulation (t3), MEP amplitudes increased initially and then plateaud at 0.55 mV (at 130% RMT) with the increase in %RMT to the right brain. Alternatively, an increase in stimulus intensity to the left brain initially increased MEPs and then led to their decrease at 130% RMT (Figure 4).
Relationship between stimulus intensity (%RMT) and MEP amplitude (mV) following stimulation of the right and left brain in A& B) patient A and C & D) patient B.
Discussion
In this study, sham tsDCS induced a slight improvement in walking speed, muscle strength, balance, and functional ability of both patients (A) and (B). A possible explanation might be that repetitive motor learning, via locomotor training, improves these functional mobility measures.2 However, sham stimulation showed no effect on spasticity which indicates that sham has no effect on the supraspinal inhibitory mechanisms of muscle tone. Spasticity has been used as a means to support and help with functional activities such as standing and walking.7 This explains how an increase in spasticity on the MAS led to an increase in functional ability following anodal stimulation and also highlights that anodal stimulation modulated the supraspinal inhibitory mechanisms of muscle tone. Moreover, cathodal stimulation also modulated the supraspinal inhibition of tone in an opposite way. Surprisingly, following multiple sessions of cathodal stimulation (t2), functional ability, represented by SCIM- III scores, did not deteriorate despite a decrease in spasticity on the MAS. This might be attributed to the repetitive execution of gait training, which led to an increase in functional ability. It is possible that the decrease in spasticity did not reach the threshold for deterioration of functional activities.
Anodal stimulation increased muscle strength whereas cathodal stimulation decreased muscle strength which is consistent with other studies.6 Despite the fact that anode and cathode tsDCS showed opposing effects, findings indicated that repetitive motor learning has a significant impact on the functional mobility measures. Interestingly, in terms of corticospinal excitability, an increase in MEP amplitudes was observed following right- and left-brain post-sham (t0) and post-cathodal (t2) stimulations. These findings are in line with findings from animal studies that showed cathodal spinal stimulation can modulate neurotransmitter release at the spinal cord and elicit changes at a supraspinal level.8 The increase in MEPs post-sham might be due to repetitive practice of the functional walking task which eventually increased corticospinal output through neuroplastic changes. It is noteworthy that while sham stimulation increased corticospinal excitability with the increase in %RMT, anodal stimulation increased MEPs only to a point after which MEPs deteriorated. However, this observation is not surprising as previous studies have observed similar results.5 Previous evidence has highlighted the effect of anodal tsDCS in prompting a hyperpolarization block to the impulses of descending cortical pathways.9 Therefore, in line with previous studies,10 it can also be concluded that tsDCS modulates ascending and descending spinal pathways and affects lower extremity functional measures in patients with SCI.
In conclusion, transcutaneous spinal direct current stimulation can be used, as a therapeutic technique, in combination with other interventions to induce neuroplastic after-effects changes and promote functional outcome measures such as reducing muscle tone, improving balance, and gait characteristics in patients with incomplete SCI.5 Combining tsDCS with gait training appears to enhance lower extremity motor function. Anodal tsDCS improves lower extremity muscle strength and increases muscle tone, which may subsequently contribute in enhancing overall locomotor ability and independence level. On the other hand, cathodal tsDCS can be used to decrease spasticity in patients with incomplete SCI. Future work should be done with a bigger sample size to investigate changes in the functional abilities of patients with SCI. Moreover, the effect of the application of tsDCS on different chronicity of SCI should be assessed.
Acknowledgment
The authors are thankful to Mr. Abdullah Alghazwani, Department of Physical Therapy, College of Applied Medical Sciences, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia, for his great help in this work. Authors would like to thank Scribendi, the Editing and Proofreading Services for English Documents (https//www.scribendi.com) for the English language editing services.
Footnotes
Disclosure. Authors have no conflict of interests, and the work was not supported or funded by any drug company.
- Received July 6, 2019.
- Accepted December 4, 2019.
- Copyright: © Saudi Medical Journal
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.