Abstract
Objectives: To evaluate the effects of platelet-rich plasma (PRP) on promoting neural repair after facial nerve compression in rats and the mechanism by which this occurs.
Methods: Adult Wistar rats (n=100) were divided into 3 groups: healthy controls, surgery-only, and surgery+PRP groups. The rats underwent nerve crush injury to establish a facial palsy model. The blood from the rats was used to prepare the PRP for application to the injury site. The evaluation methods included vibrissae movement, eyelid closure, and electrophysiology. Electron microscopy, immunohistochemistry, and real-time polymerase chain reaction (PCR) were used to detect nutrient factor expression in the brain and nerve sections. This study was conducted in Shandong Provincial ENT Hospital Affiliated to Shandong University, Shandong, China between January and November 2018.
Results: Platelet-rich plasma promotes the recovery of vibrissae movement, eyelid closure, and electrophysiological function in a rat model of nerve crush injury. Hematoxylin and eosin staining, toluidine blue staining, and electron microscopy showed significant recovery of Schwann cells and axons in the PRP group. Polymerase chain reaction results showed that PRP releases growth factors, which include nerve growth factor and brain-derived neurotrophic factor. Immunohistochemistry also demonstrated higher levels of S-100 protein expression in the PRP group compared to the other groups.
Conclusions: Platelet-rich plasma releases nutrient factors in the brainstem, and the use of PRP can promote injury recovery.
Facial expressions are evolutionary adaptations that promote successful social interactions. The muscles in facial expression are essential for nonverbal communication and joint and corneal protection.1 Facial paralysis leads to functional, communicative, and social disorders and has a profound negative effect on the quality of life and emotional well-being. Traumatic facial palsy is very common and can be caused by traffic accidents, intratemporal or extratemporal surgery including parotid gland surgery and middle ear surgery.2 The facial nerve plays an important role in life, and facial nerve damage can affect aesthetics and even cause psychological problems.3 Although there has been great progress in the medical treatment of facial nerve injury, the functional recovery achieved is still inadequate. Injury of the facial nerve induces the brainstem to release nutrient factors and promotes repair of the nerve. Therapies for nerve injury include systemic or topical medication, nerve anastomosis, nerve transplantation, neurotrophic drugs, acupuncture, and moxibustion therapy.4 The pathology of nerve injury is classified into 5 grades:5 nerve conduction block, axon interruption, endoneurial nerve disruption, membranous nerve disruption, and complete nerve disruption. In this study, the grade of nerve crush injury was considered to be between axon interruption and endoneurial nerve disruption.
As part of a new generation of biological products, platelet-rich plasma (PRP) contains several growth factors including nerve growth factors, transforming growth factors, and platelet-derived growth factor.6 Platelet-rich plasma is obtained by the centrifugation of blood and is used in many medical areas such as traumatic orthopedics, plastic surgery, urological surgery, and neurosurgery.7 Platelet activation can release various trophic factors that play crucial roles in injury. These factors enhance remyelination and regeneration in the nervous system. Platelet-rich plasma is widely used in many fields such as otolaryngology8 to improve healing times after tympanoplasty, tonsillectomy, and functional endoscopic sinus surgery. Platelet-rich plasma is effective in facial nerve injury; however, the mechanism is not well understood.9
Therefore, this study evaluated the morphological and biological effects of PRP in facial nerve crush injury. To this end, we established a rat model of facial nerve crush injury, and the injury + PRP group was compared with the control group after nerve crush injury. In addition, the neuroprotective effects of PRP on facial nerve injury in rats were investigated, specifically the effects on functional recovery and pathologic changes. The results demonstrated the morphological and biological effects of PRP in facial nerve crush injury and showed evidence of PRP in nerve repair.
Methods
A search of studies on PRP treatment in nerve injury was performed in PubMed (https://www.ncbi.nlm.nih.gov/pubmed/) to find prior related research.
Animals
Female Wistar rats (average weight, 210 g; n=100) were used in the study and were supplied by the Shandong University Animal Center (Jinan, China). All animals were housed in a standard laboratory under a 12 hours/12 hours natural light-dark cycle, and clean food and drinking water were provided. Animal use was approved by the Animal Ethics Committee of Shandong University.
Experimental design
The 100 rats were divided into 3 groups: control group (normal rats, n=10), injury group (n=40), injury+PRP group (n=40); the remaining rats (n=10) were used to supply blood for the PRP group. Six injury and 6 injury+PRP rats were used to assess facial expression scores for one month (recorded every 3 days for eye blink reflex and vibrissae movement; Table 1), and the rats were sacrificed after one month. Electromyography was performed in vivo, the facial nerve of the rats was removed for pathological sectioning, and the sections were stained using hematoxylin and eosin (H&E) and toluidine blue. The remaining rats were selected for the 1-week, 2-week, and 3-week model groups (each group contained at least 4 injury and 4 injury+PRP rats). On days 7, 14, and 21, electrophysiological readings were recorded and the rats were sacrificed. The injured facial nerves and facial nucleus of the brainstem were removed for immunohistochemistry (IHC) and PCR, respectively.
Evaluation of facial nerve function.
Platelet-rich plasma preparation
Ten rats were used to obtain blood for PRP. The animals were anesthetized by injection of chloral hydrate (400 mg/kg) into the abdominal cavity and blood was collected via cardiac puncture.10 Fresh blood (5 mL) was collected in a sterile tube and 10% sodium citrate was added. A 2-step centrifugation was performed (10 min, 200 g/min followed by 300 g/min for 15 min).11 The platelet density was 900×109 platelets/L and a total of 0.5 mL PRP was collected for every 5 mL fresh blood. Platelet-rich plasma gel was obtained by mixing PRP with thrombin (Shanghai Shenggong Trade Co. Ltd., Shanghai, China) at a 10:1 ratio. Platelet-rich plasma was collected and stored at -80°C until subsequent use. Platelet-rich plasma was mixed with 100 U/mL thrombin (1:0.15 ratio) and 10% CaCl2 (Shanghai Shenggong Trade Co. Ltd.) for platelet activation.
Animal groups and crush injury
The animals were anesthetized by injecting 0.8 mL of 400 mg/kg chloral hydrate (Shandong Provincial ENT Hospital, Jinan, China) into the abdomen. The left posterior auricular skin of the rats was shaved and disinfected with alcohol. The circumferential incision around the posterior groove of the external auditory canal was approximately 1 cm in length, and the muscle fascia was obtusely separated.12 Then the facial nerve at the stylomastoid foramen was exposed and the facial nerve was crushed twice for 30 seconds (10 seconds interval) using Dumont forceps. For the injury group, the wound was immediately sutured. For the injury+PRP group, PRP gel was applied and a syringe with approximately 0.5 mm PRP was injected into the wounded area every 4 days. The animal care and experimental use program was agreed upon by the Animal Care Committee of Shandong University (No. 20173011).
Behavioral and electrophysiological study
Two variables were observed: the eye-blink reflex and vibrissae movement. Vibrissae movements were recorded by comparing the injured side to the normal side, using a 5-point scale, as described by Farrag.12 The eye-blink reflex was evaluated by blowing air into the eye. A 50 mL syringe (without needle) was situated approximately 2 cm from the eye and was used to blow air into the eye, as described by Borin.13 Electromyography recordings were performed on days 7, 14, and 21 after surgery at Shandong Provincial ENT institute (Jinan, China). Two stimulating electrodes were inserted at the intersection of the canthus vertical line and the surface of the buccal branch of the facial nerve,14 and the collecting electrode was inserted subcutaneously on the left side of the orbicularis oris muscle. A reference electrode was inserted subcutaneously on the skull. The compound muscle action potentials (CMAPs) of the injured facial nerves were analyzed. In the process of inducing facial nerve CMAPs, the first significant wave was the M wave.15 The initial latency and amplitude were recorded. The maximum stimulation of the facial nerve was 5 mA, and the peak amplitude and latency were recorded. Normal CMAP values were obtained from the control group.
Histological observations of nerve regeneration
Anesthetized rats were sacrificed after testing facial function. Then the distal segment of the injured facial nerve was immediately removed, immersed in 4% paraformaldehyde (PFA) for 3 hours, and sent to Jinan WeiYa Bio-Technology Co. (Shandong, China) for electron microscopy. Facial nerves from each group were stained with 1% toluidine blue 4 weeks after surgery to examine their appearance by histology. The remaining nerve tissues were dehydrated with 25% sucrose overnight at 4°C and were later embedded in paraffin. Cross-sections (6 mm thick) were collected on glass slides. Some tissue sections were stained with H&E. The cross-sections were dehydrated in graded ethanol solutions and stained with hematoxylin for 5 min and then eosin for 3 min. The sections were washed with double-distilled water and then dehydrated with gradient ethanol solutions. We used facial nerve trunks from 5 mm distal to the lesion site for light microscopy observation.
mRNA extraction and quantitative PCR
On days 7, 14, and 21 after facial nerve injury, the rats were anesthetized, sacrificed, and the facial nucleus of the brainstem and injured nerve segment were removed. Total RNA of the facial nucleus and injured nerve segment was extracted.12 The nucleus and tissue segment were washed with 1× phosphate-buffered saline (PBS) and immediately lysed in TRIzol (Invitrogen, Gaithersburg, MD, USA) according to the manufacturer’s protocol. The 25 µL quantitative PCR (qPCR) reaction consisted of 12.5 µL SYBR Green Premix EX Taq (TaKaRa, Dalian, China), 0.5 µL forward primer, 0.5 µL reverse primer, 1 µL cDNA template, and 10.5 µL deionized water, The cDNA was synthesized by reverse transcription of 1 µg total RNA using the ExScript RT Reagent kit (TaKaRa, Dalian, China). The PCR conditions were as follows: pre-denaturation at 95°C for 3 min, denaturation at 95°C for 45 seconds, annealing at 60°C for 35 seconds, extension at 70°C for 50 seconds and final extension at 70°C for 5 minutes. The cDNA of b-actin (internal control), brain-derived neurotrophic factor (BDNF), and nerve growth factor (NT-4) were amplified for 28 cycles with their respective primers (Table 2).
Polymerase chain reaction primer sequences used for quantitative polymerase chain reaction (qPCR).
Longitudinal facial nerve sections for IHC
After electromyography recordings were performed, the facial nerve proximal segment was removed from the 1-week, 2-week, and 3-week model rats. The nerve tissues were immersed in 4% PFA for 3 hours, dehydrated with 30% sucrose overnight at 4°C, and then embedded in optimal cutting temperature compound (OCT compound) gel. The -20°C freezing microtome was used to make continuous 5 µm longitudinal sections that were stored at -80°C. The frozen slices were warmed in a 37°C oven for 1 hour and incubated in 0.3% PBS with Tween® detergent to rupture the cell membrane,16 followed by 3% H2O2 for 30 min to quench endogenous peroxidase activity. The sections were later incubated in 10% normal goat serum (diluted in PBS) for 30 min, followed by polyclonal rabbit antibody targeting S-100 (Ab14849, 1:3000; Abcam, NY, USA) at 4°C for 10 hours, and the appropriate secondary antibody for 30 minutes. The peroxidase reaction product was visualized. All sections were counter stained with hematoxylin and mounted for examination.
Statistical analysis
The data were analyzed using Statistics for Windows, version 17.0 (SPSS Inc., Chicago, IL, USA). All values were expressed as mean ± standard error of the mean. Statistical analysis of the facial scores was performed by one-way analysis of variance. The PCR statistics were calculated by the t-test. P values <0.05 were considered statistically significant.9
Results
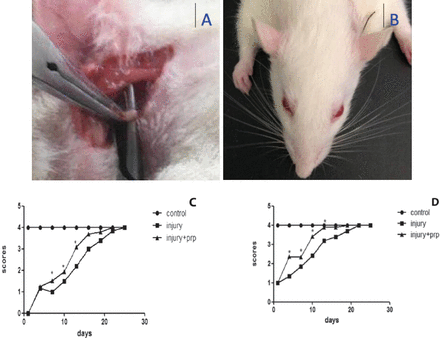
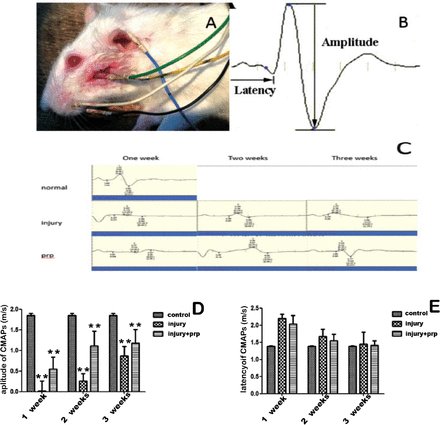
After nerve crush injury, facial palsy was successfully induced in all rats. Blink reflexes and vibrissae movements were carefully observed every 3 days to detect and evaluate facial functional recovery. Within 28 days, the facial paralysis was healed naturally (Figure 1). The recovery time of the PRP group was shorter than that of the injury group, and the difference was statistically significant (p<0.01). The normal latency of the rat facial nerve compound muscle action potentials (CMAPs) was 1.38±0.01 ms and the amplitude was 1.85±0.05 mV. After surgery, the latencies of the 6 rats in the injury group and the 6 rats in the injury+PRP group were markedly prolonged compared with those in the control group, and the amplitudes of the PRP group showed improvement at 3 weeks compared with the injury group (Figure 2).
Surgical position and time course of nictitating reflex and vibrissae movement recovery. A) The facial nerve was exposed, and the trunk was crushed using vessel forceps. B) A photo showing facial palsy on the left side. The left nose is shifted to the right and the left eye is unable to close. The time course of C) vibrissae movement and D) blink reflex recovery. The rats in 2 of the groups exhibited the onset of transient facial paralysis 4 weeks after operation. The facial function of the rats in the second and third groups returned to normal. However, the facial function of the platelet-rich plasma (PRP) group was significantly better than that of the injury group. *p<0.05 versus injury group.
Electrophysiological evaluation of compound muscle action potentials. A) Each electrode line was inserted at a different position. B) Diagram of the amplitude and latency of a CMAP. C) Three groups of animals were subjected to electromyography 7, 14, and 21 days after surgery. Representative data are shown for each group of 3 rats. The incubation period on the chart is the time from nerve stimulation to muscle depolarization. CMAPs were observed in the 3 groups after surgery. Electrophysiological assessment of the facial nerves of the rats in all groups, as measured by the D) amplitude and E) latency of CMAPs at 1, 2, and 3 weeks after operation. **p<0.01. PRP - platelet-rich plasma.
Morphological changes
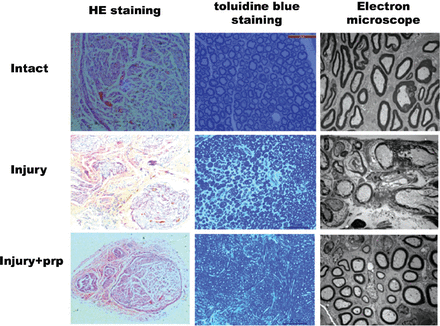
Hematoxylin staining staining of regenerated facial nerves was performed, and the morphology of the regenerated nerves was analyzed by electron microscopy. In the PRP group, the neovascularization of the nerve bundle was significantly greater than that in the injury group, and the number of vacuoles formed by nerve fiber degeneration and necrosis was significantly less than that in the injury group (Figure 3). The myelin sheaths of the facial nerves were normal in the rats.16 The synaptic cleft was large, deep, and long; however, after crush injury, most nerve fibers were lumpy, and the synaptic cleft was smoother and smaller.17 The normal facial nerves exhibited circular myelin fibers that were evenly distributed throughout the entire field. The facial nerves from the injury group showed a small number of thin and irregular myelin fibers, while those in the injury+PRP group showed increased number of thick myelin fibers.
Platelet-rich plasma increases mRNA expression of BDNF and NT-4 in the facial nerve segment and decreases mRNA expression in the nucleus.
Regenerated facial nerves stained with Haemotoxylin and Eosin (H & E) and toluidine blue and examined by electron microscopy at one month following surgery. Most blood vessels were partially degraded into the surrounding tissue without serious side effects. PRP - platelet-rich plasma
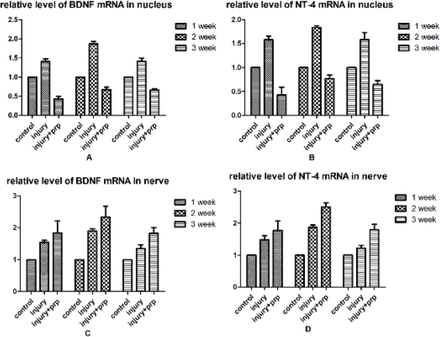
The results of qPCR showed that the PRP group exhibited a marked increase in the expression of BDNF and NT-4 in the facial segment compared to the injury group. In addition, the PRP group showed a decrease in expression of BDNF and NT-4 in the nucleus compared to the injury group. This result suggests that PRP (and not the brain) produces various trophic factors (Figure 4).
Quantitative polymerase chain reaction (qPCR) for neurotrophic factors (BDNF and NT4). A) BDNF expression in the facial nerve nucleus of 7, 14, and 21 days post-injury groups. B) NT4 expression in facial nerve nucleus of 7, 14, and 21 days post-injury groups. C) BDNF expression in the facial fragment. D) NT4 expression in the facial fragment. The results showed time-dependent changes in BDNF and NT-4 expression in the three groups after facial nerve injury. The y-axis is the relative mRNA expression level of BDNF and NT-4 Messenger RNA (mRNA). BDNF - Brain-derived neurotrophic factor, NT4 - neurotrophin 4
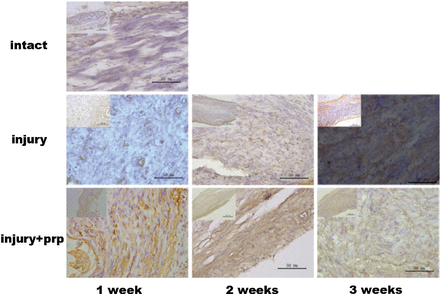
Platelet-rich plasma increases S-100 protein expression in the longitudinal nerve sections
S-100 protein expression in the facial nerves was analyzed by IHC (Figure 5). In the normal facial nerve, S-100 protein expression was negligible. At one week after crush injury, the S-100 protein expression in the facial nerve was significantly upregulated in the PRP group. In the normal facial nerve, a low amount of S-100 protein expression was observed in Schwann cells; however, S-100 was highly expressed in both nerve fiber axons and Schwann cells at 7 days after facial nerve injury. On day 21 after injury, S-100 protein expression was slightly reduced. In the treatment of neuronal injury, PRP plays a protective role by mainly inhibiting the expression of S-100, blocking its transport to the nucleus, and inhibiting transmission of the S-100 signal pathway, thereby reducing the release of inflammatory factors, alleviating the damage to nerve cells, and blocking the vicious cycle of the inflammatory response.
Immunohistochemical analysis of S-100 expression. Immunohistochemical staining showed that S-100 expression was observed in the cytoplasm of injured facial nerves. The expression of S-100 was low in the facial nerves of the control group. S-100 was highly expressed in injured facial nerves and Schwann cells. However, S-100 expression levels was decreased after 3 weeks with lower levels of S-100 observed in the injury+PRP group compared with the injury group.
Discussion
In the present study, a rat model of extracranial nerve crush injury was used, in which the crush injury occurred in the stylomastoid foramen. This study validated the role of PRP in animal models and suggested the nutritional role of plasma as an alternative to the nutrients released from brain tissue. The facial nerve injury is likely to occur in the intracranial area as there are branches extending from the far end of the trunk to this position. In addition, to reduce the cost and difficulty of establishing this animal model, extracranial facial nerve trunk near the above-mentioned site for injury was chosen. The results of this study suggest that PRP induces redifferentiation after facial nerve crush injury. Injury of the nerve trunk led to dissymmetry in the blinking reflex and whisker movement, which indicated successful establishment of a model of facial paralysis. Based on the facial scores and electrophysiological recordings, the PRP group recovered within a shorter time than the normal group, indicating that PRP treatment promoted the recovery of facial nerve function after facial nerve injury. We searched for PRP effects in PubMed, and showed that it can promote growth.
In this study, the electrophysiological manifestation in rats was the nerve conduction block, which is reversible. The electrical excitation threshold of normal fibers is nearly equal to that of fibers with blocked conduction; however, the response to electrical stimulation is significantly reduced in denatured nerve fibers.17 The rats in the PRP group exhibited a shorter latency time and a higher amplitude in compound action potential compared with the surgery group. Excitation of the nerve fibers due to electrical stimulation causes the facial muscles to contract. The extent of degeneration of nerve fibers on the side of the lesion compared with normal CAMPs can be used to judge the degree of neuropathy.10 A CAMP is a reflex electromyogram of a specific waveform, and it has an amplitude and latency. The waveform amplitude depends on the number and size of discharged fibers. The speed of neural transmission is proportional to the size of the fibers, the formation of myelin, and the internodes.18
In this study, the crush injury was mild, and the 4-week model exhibited almost full recovery. In addition, the morphologic evaluation of the injured facial nerves demonstrated the basic pathologic changes of facial crush injury. The nerve axons were pinched under uneven pressure. After 4 weeks, the second group exhibited a small number of proliferating Schwann cells, with myelin and edema beginning to recover; however, the nerve fibers remained sparse and uneven. The third group exhibited a large number of proliferating Schwann cells accompanied by inflammatory cell infiltration and myelin and edema recovery. Based on histological observations, myelin was most sensitive to nerve damage. The more severe damage was manifested as degeneration observed by electron microscopy.19 Denatured myelin sheaths were disintegrated to produce spherical medullary changes, while some denatured myelin sheaths were degenerated into annular bodies to compress axons; however, the endothelial canal was intact. Haemotoxylin and Eosin staining was used to identify edema, hyalinization, and inflammatory cell infiltration after facial nerve injury.20 Following nerve injury, a cascade reaction occurs, followed by neuronal degeneration and death. Platelet-rich plasma has the potential to promote the proliferation and differentiation of neuroglial cells, provide the micro-environment needed for the growth and development of regenerating axons, and promote nerve regeneration and functional recovery. This has also been confirmed by electron microscopy.18 S-100 are specific peptides or proteins derived from neurons,19 which play important roles in regulating survival. It has been shown that S-100 can promote the outgrowth of neurites and the survival of neurons. In this study, IHC was conducted to examine S-100 in Schwann cells and the results showed high levels of S-100 protein expression in rats treated with PRP, whereas this was not observed in rats in the surgery alone group. Platelet-rich plasma may play a role in promoting remyelination, prevent the apoptosis of nerve cells, maintain the normal functions of neurons, and promote regeneration after nerve injury. In addition, PRP can increase the rate of axonal regeneration after crush injury.12 A faster recovery time for vibrissae motion and blink reflex demonstrated the role of PRP in recovery and further confirmed by electrophysiology. Immunohistochemistry showed accelerated growth of axons, while PCR showed increase in expression of neurotrophic factors.
Platelet-rich plasma has recently gained popularity as a treatment for a variety of tissues in almost all fields of surgery,18 as research on PRP has demonstrated the important neurotrophic effects of PRP on regeneration after facial nerve crush injury in a rat model. This study also observed a significant increase in expression of neurotrophic factors (BDNF and NT-4) in the facial nerve fragment. In addition, BDNF and NT-4 genes were highly expressed in injury+PRP groups, suggesting that PRP may act as a source of neurotrophic factors. On the other hand, decreased expression of neurotrophic factors was observed in the facial nucleus, and its function could be partially inhibited by PRP. It is known that PRP promotes wound healing; however, the mechanism is currently unknown. This study showed that the nutrients released from platelets can be used to replace nutrients released from the brainstem. These findings may pave the way for future research on accelerated healing.
Study limitations
The research was performed in animals and has yet to be tested in humans; and Western blotting was not performed to validate the PCR results.
In conclusion, this study provides evidence that PRP has neuroprotective effects against facial nerve crush injury in rats. These effects may be associated with the release of neurotrophic factors induced by PRP to promote facial nerve repair. Thus, the use of PRP may be a promising strategy for alternative therapy for facial nerve injury in the future.
Acknowledgment
The study was supported by the National Natural Science Foundation of China (No. 31700894//C0907), Beijing, China. We would like to thank Medjaden Bioscience Limited for English language editing.
Footnotes
Disclosure. The authors declare no conflicts of interest. The study was supported by the National Natural Science Foundation of China, Beijing, China (Grant No. 31700894/C0907).
- Received July 4, 2019.
- Accepted November 11, 2019.
- Copyright: © Saudi Medical Journal
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.