Abstract
A 62-year-old woman underwent a right mastectomy with axillary node dissection for a poorly differentiated ductal carcinoma. One month later, she underwent a left nephrectomy for a renal cell carcinoma. Two weeks after, she received her first cycle of cyclophosphamide, methotrexate, and 5FU (CMF) as a part of her breast cancer treatment. We describe an unusual case of non-occlusive saddle pulmonary embolus with extensive bilateral deep vein thrombosis and severe bronchiolitis obliterans with organizing pneumonia developing simultaneously after the first CMF chemotherapy for breast cancer.
Pulmonary embolism (PE) is a serious condition that commonly develops in hypercoagulable states such as malignancy and post chemotherapy treatment.1 When associated with hemodynamic instability, immediate recognition, and treatment are essential for a favorable outcome.2 Elevated pulmonary artery pressures (PAP) and pulmonary vascular resistance (PVR) may be the clue to diagnosis and subsequently the treatment of PE.3 Bronchiolitis obliterans with organizing pneumonia (BOOP) is another serious disease that is frequently misdiagnosed and is associated with significant morbidity and mortality. Early recognition and treatment usually leads to better outcomes.4 The objective in presenting this particular case is to describe a rare case of significant pulmonary embolism and severe BOOP developing simultaneously after the first cyclophosphamide, methotrexate, 5FU (CMF) chemotherapy for breast cancer.
Case Report
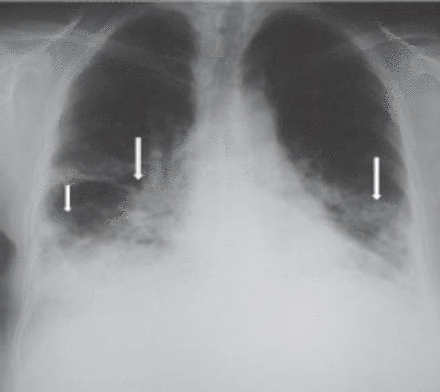
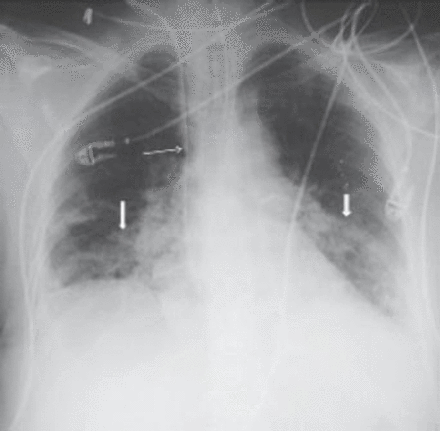
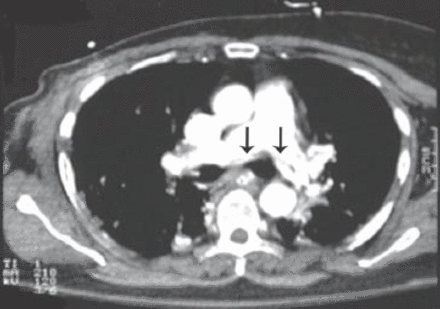
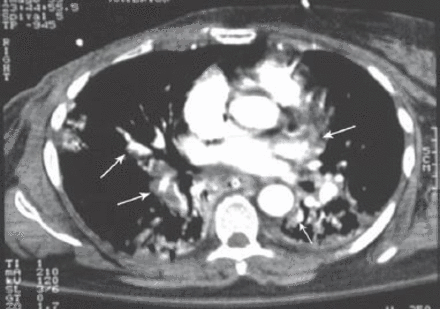
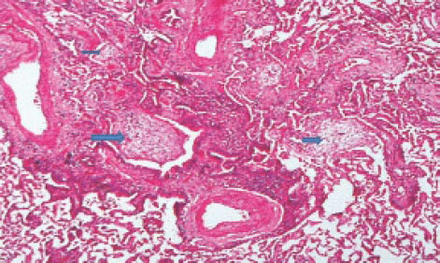
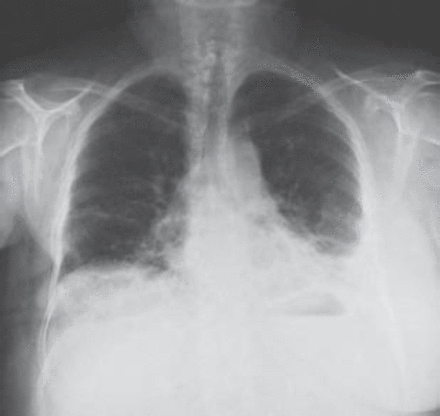
A 62-year-old woman underwent a right mastectomy with axillary node dissection for a poorly differentiated ductal carcinoma. One month later she underwent left nephrectomy for renal cell carcinoma. After 14 days, she received her first cycle of CMF as a part of her breast cancer treatment. Two weeks later, she developed progressive shortness-of-breath, dry cough, and mild fever, for which she was started on antibiotics for possible pneumonia. Two days later, her respiratory status deteriorated and she required intubation and mechanical ventilation. Because of hemodynamic instability and hypotension, she was started on inotropes and transferred to the intensive care unit. On examination, her blood pressure was 110/70 mm Hg, pulse of 104 beats per minute, normal temperature, and her jugular venous pressure was 8 cm above the sternal angle. Chest examinations revealed bilateral bronchial breathing and diffuse crackles. Cardiovascular and abdominal examinations were unremarkable. Her initial chest x-ray showed bilateral mixed alveolar and interstitial infiltrates with volume loss (Figure 1). Her arterial blood gases on room air prior to intubation, were partial pressure of oxygen 47torr, partial pressure of carbon dioxide 32torr, pH 7.49, bicarbonate 21, O2 saturation was 85%. The PAP was 36/21 mm Hg, pulmonary capillary wedge pressure 12 mm Hg, central venous pressure 20 cm H2O, cardiac index of 2.0 L/min/m2, and calculated PVR was 560 dynes sec/cm-5 (Figure 2). A spiral CT of the chest showed a non-occlusive saddle pulmonary embolism with multiple bilateral pulmonary emboli (Figures 3 & 4). She was treated with thrombolytics (100 mg rt-PA) followed by heparin without complications. She improved hemodynamically, and the inotropes were discontinued. Doppler ultrasound of the legs confirmed bilateral deep vein thrombosis. Because of persistent pulmonary infiltrates (Figures 1 & 2), she underwent a fiberoptic bronchoscopy with bronchoalveolar lavage, which was negative. This was followed by open lung biopsy that showed loose organizing granulation tissue in the small airways consistent with BOOP (Figure 5). She was treated with a pulse of steroids (1 g of methylprednisolone intravenously daily for 3 days followed by 1 mg/kg of prednisone daily). She was extubated 8 days later. She was transferred to the medical ward and was discharged home on long-term anticoagulation and steroids 2 weeks later. She had a chest x-ray before discharge, which showed near resolution of the infiltrate and restoration of lung volumes (Figure 6).
Initial chest x-ray showed bilateral mixed alveolar and interstitial infiltrates.
Chest x-ray after intubation and pulmonary artery catheter insertion, showed endotracheal tube and pulmonary artery catheter in good place with bilateral air space.
Spiral CT scan of the chest showed a non occlusive saddle pulmonary embolism.
Spiral CT scan of the chest showed multiple bilateral pulmonary emboli and bilateral air space.
Arrows show loose organizing granulation tissue in small airways consistent with an organizing pneumonia pattern of cryptogenic organizing pneumonia or bronchiolitis obliterans with organizing pneumonia.
Chest x-ray before discharge, which showed near resolution of the infiltrate and restoration of lung volumes.
Discussion
Massive PE is a catastrophic entity that often results in acute right ventricular failure, hemodynamic instability, and death.1 The diagnosis of massive PE relies mainly upon clinical suspicion followed by the appropriate investigation.2 Spiral CT of the chest is the diagnostic modality that is most often used in ventilated patients, although pulmonary angiogram remains the gold standard.3 Clinical instability may interfere with the institution of an effective diagnostic strategy, and could therefore delay early therapy. In our case, pulmonary artery catheter data led to the suspicion of PE and subsequent appropriate investigation and early therapeutic intervention. Thrombolysis is currently the standard treatment of massive PE with hemodynamic instability.2
Bronchiolitis obliterans with organizing pneumonia is another distinct clinical entity of unknown etiology. It is also called cryptogenic organizing pneumonia, and is defined pathologically by the presence in the distal air spaces of buds of granulation tissue progressing from fibrin exudates to loose collagen containing fibroblasts.4,5 It is commonly presented with pneumonia-like symptoms that do not respond to antibiotics. Organizing pneumonia (BOOP) may be classified into 3 categories according to its cause: organizing pneumonia of determined cause, organizing pneumonia of undetermined cause but occurring in a specific and relevant context, and cryptogenic (idiopathic) organizing pneumonia. Several possible causes and/or associated disorders may co-exist in the same patient. There are no clear distinguishing clinical and radiological features between cryptogenic and secondary organizing pneumonia.5,6
With drug-induced organizing pneumonia, it is sometimes difficult to determine causality since organizing pneumonia may also be associated with the underlying disease. In many cases however, it is not possible to determine whether the drug is responsible or not, for example, in patients with cancer or hematological malignancies who may be treated with several drugs that are able to induce organizing pneumonia.6 Bleomycin and busulphan are the most common chemotherapy drugs associated with BOOP. However, on MEDLINE review, we were unable to source CMF described in the literature as a cause of BOOP after the first cycle of chemotherapy. Chemotherapy, particularly cyclophosphamide, has been used as a second line treatment after steroids for BOOP. Though cyclophosphamide and methotrexate treatment have been rarely reported in the literature to cause BOOP, 5-FU has not been reported to cause BOOP.7,8 Systemic corticosteroid therapy is the mainstay of treatment with initial doses of 0.75 to 1 mg/kg per day, and gradual weaning over 6 to 12 months.9 Because of her respiratory failure requiring mechanical ventilation, our patient received a pulse of steroids over 3 days followed by 1 mg/kg of prednisone daily.10
This case highlights the importance of using the new chemotherapy regimen for breast cancer with fewer side effects including lung toxicities. In our case, BOOP was suspected after ruling out active infection and following an adequate course of antibiotic coverage. Open lung biopsy provided the definite diagnosis. Corticosteroids remain the mainstay of therapy.
In conclusion, multiple primary causes for respiratory failure in a single patient are not uncommon. In a patient at risk for PE, the diagnosis should be pursued further in the presence of unexplained high PAP and PVR. Bronchiolitis obliterans with organizing pneumonia is being increasingly diagnosed as a cause of respiratory failure and should, in the classical setting be commonly included in the differential diagnosis. The presence of an evident cause for respiratory failure should not deter further investigation.
Related Articles
Thabet FC, Alhejaili AS, Alodayani AN, Chehab MS. Septic pulmonary embolism secondary to Staphylococcus aureus septic thrombophlebitis in a pediatric patient. Saudi Med J 2013; 34: 1080-1082.
Darwish HS, Qamar SR. Paddlewheel multi-slice helical computed tomography reformation in the detection of pulmonary embolism. An initial experience. Saudi Med J 2013; 34: 896-900.
El-Essawy MT, Al-Harbi SR. Multiple pulmonary and systemic ectopic emboli following endoscopic injection sclerotherapy for gastric fundal varices. Saudi Med J 2012; 33: 321-323.
Footnotes
Disclosure. The author has no conflict of interests, and the work was not supported or funded by any drug company.
- Received January 22, 2015.
- Accepted April 27, 2015.
- Copyright: © Saudi Medical Journal
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.