Abstract
Objectives: To study the associations between hyperhomocysteinemia (HHcy) and the severity of coronary heart disease (CHD).
Methods: We retrospectively analyzed metabolic parameters, anthropometric variables, and life style habits in 292 CHD patients of different categories, and 100 controlled non-CHD patients with chest pain symptoms who were hospitalized in the Department of Cardiovascular Medicine, Sichuan Academy of Medical Sciences and Sichuan Provincial People’s Hospital, Chengdu, China between October 2013 and September 2014.
Results: The prevalence of HHcy in CHD patients was 79.1%, while only 5% of non-CHD patients had elevated serum homocysteine (Hcy) concentrations. The prevalence of HHcy significantly increased from 5% in non-CHD controls to 66% in the stable angina pectoris (SAP) group, to 81.9% in the unstable angina pectoris group, and to 93.15% in the acute myocardial infarction (AMI) group (p<0.001). After adjusting for confounding factors, multivariate logistic regression analysis showed that HHcy was independently associated with CHD category (AMI versus SAP, odds ratio [6.38], 95% confidence interval; 1.18-34.46). The Hcy was negatively correlated with folic acid (r=-0.67, p<0.001) and vitamin B12 (r=-0.56, p<0.001). Of the CHD patients with HHcy, 51.1% had low folic acid and 42% had low vitamin B12, 7 or 5 times higher than that of CHD patients with normal-low Hcy concentrations (p<0.001).
Conclusion: Hyperhomocysteinemia is independently associated with the severity of CHD, and significantly correlated with low status of folic acid and vitamin B12 in CHD patients.
Coronary heart disease (CHD) is the greatest single cause of mortality and loss of disability-adjusted life years worldwide, and a substantial portion of this burden falls on low- and middle-income countries.1,2 Despite best efforts, available therapies protect only 30-40% of individuals at risk, and no therapeutic cure is anticipated for those who currently suffer from the disease.3 Research has confirmed that endothelial dysfunction plays a pivotal role in all phases from the initial formation of fatty streaks to vulnerable plaque rupture of atherosclerosis, which leads to serious complications in CHD patients, and is reversible at every phase, indicating that endothelial function-guided therapies might be effective and feasible in cardiovascular practice.4 The severity of atherosclerosis with endothelial dysfunction in CHD patients is continuously changing and progressively increases in pathological process from stable angina pectoris (SAP) to unstable angina pectoris (UAP), and to acute myocardial infarction (AMI).5 However, early warning and immediate risk stratification for the severity of CHD patients is frequently a challenging task currently. Detecting changes of metabolic parameters, anthropometric variables and life style habits in CHD patients with different categories may be provide more important information to early warning and risk stratification. There is strong evidence indicating that elevated serum homocysteine (Hcy) has been associated with endothelial dysfunction of atherosclerosis, and hyperhomocysteinemia (HHcy) has been considered an independent risk factor for cardiovascular disease.6 Folic acid and vitamin B12 as cosubstrate and cofactor, are 2 vital regulators in the metabolic process of Hcy.7 In addition, current studies have shown that folic acid supplementation can significantly improve endothelial dysfunction in patients with CHD.8 It has also been reported that serum vitamin B12 deficiency and HHcy are related to cardiovascular risk factors in patients with CHD.9 A better understanding of the association between HHcy and the severity of CHD, as well as the correlations of Hcy with folic acid and vitamin B12 may be a new strategy for early warning and risk stratification in CHD patients. In the present study, we retrospectively analyzed the metabolic parameters and anthropometric variables, as well as life style habits in 292 CHD patients of different categories, and 100 control non-CHD patients with chest pain symptoms. Our objective was to explore the possible associations between HHcy and the severity of CHD, and evaluate the correlations of Hcy with folic acid, as well as vitamin B12 in patients with CHD.
Methods
A total of 292 CHD patients aged 36-85 years hospitalized in Department of Cardiovascular Medicine, Sichuan Academy of Medical Sciences & Sichuan Provincial People’s Hospital, Chengdu, China between October 2013 and September 2014 were included in this study. All enrolled CHD patients had been confirmed by coronary angiography and included 73 with AMI, 116 with UAP, 103 with SAP according to 2007 ACC/AHA guidelines10 One hundred non-coronary heart disease (non-CHD) patients aged 35-83 years with chest pain symptoms during the same period were confirmed by coronary angiography. Patients with the following diseases were excluded from this study: cancer, liver diseases, renal insufficiency, blood diseases, hyperthyroidism, thyroid dysfunction, systemic lupus erythematosus, malnutrition, pregnant woman, and supplemented folic acid, and vitamin B12. This study was carried out in accordance with the Helsinki Declaration and approved by the Regional Ethics Committee. All participating subjects were advised of their participation rights and written informed consent was obtained. They were questioned regarding their life style habits of alcohol intake (yes or no, it was defined as yes at least once a week and drinking over 45% by volume alcohol, more than 200 mL), and smoking (yes or no, non-smokers including never smoking, and stop smoking more than one year). Hypercholesterolemia and hypertriglyceridemia were defined as total cholesterol (TC) ≥6.22 mmol/L, and triglycerides (TG) ≥2.26 mmol/L, according to the 2007 China Adult Dyslipidemia Prevention Guide.11 Hypertension was diagnosed when patients’ systolic blood pressure (SBP) ≥140 mmHg and/or diastolic blood pressure (DBP) ≥90 mmHg. Overweight and obesity was defined as body mass index (BMI) (24.00-27.99) kg/m2, and ≥28.00 kg/m2, according to the 2006 Guidelines for Prevention and Control of Overweight and Obesity in Chinese Adults.13 Data was consecutively collected from the Sichuan Academy of Medical Sciences & Sichuan Provincial People’s Hospital, from October 2013 to September 2014. Fasting blood was sampled in the morning after 12-15 hours fasting, and after the patient had been sedentary in a sitting or supine position for at least 15 minutes. The blood of all subjects was collected before the heparinization of coronary angiography. The blood of AMI patient was collected before percutaneous coronary intervention and thrombolytic treatment. Sampled blood was transferred, ensuring flow down the wall of the tube, and never directly squirted into the center in order to minimize the mechanical disruption or turbulence, which could be result in hemolysis or activation. The Hcy was measured by enzymatic cycling assay with an Hitachi 7600 Automatic Biochemistry Analyzer (Hitachi High-Tech Instruments Co., Ltd., Hitachinaka City, Ibaraki-ken Japan; reagent and calibrator, Beijing Strong Biotechnologies, Inc., Beijing, China; quality control material of Liquichek™ Hcy, Bio-Rad Laboratories, Inc., Irvine, CA USA). The HHcy was defined as greater than 15 µmol/L of serum Hcy level according to the reference interval with 5-15 µmol/L. The cut-off values of CHD patients for tertiles distribution of Hcy were <17.31 µmol/L for I tertile, 17.31-24.64 µmol/L for II tertile, and > 24.64 µmol/L for III tertile. The levels of folic acid and vitamin B12 were quantified by microparticle chemoluminescence immunoassay with ACCESS 2 Immunoassay System and matched reagent, calibrator and quality control materials (Beckman Coulter, Inc., Chaska, MN, USA). The low folic acid and vitamin B12 were defined as less than 6.8 nmol/L of folic acid level, and smaller than 133 pmol/L of vitamin B12 concentration value according to the reference intervals, which were ≥6.8 nmol/L for folic acid and 133-675 pmol/L for vitamin B12. The cut-off values of CHD patients for tertiles distribution of folic acid and vitamin B12 were: I - <5.73 nmol/L for folic acid, and 113 pmol/L for vitamin B12, II - 5.73-9.67 nmol/L for folic acid, and 113-364 pmol/L for vitamin B12, and III - >9.67 nmol/L for folic acid, and 364 pmol/L for vitamin B12. The concentrations of TC, TG, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), glucose (GLU) and uric acid (UA) were measured with Hitachi 7600 Automatic Biochemistry Analyzer (Hitachi High-Tech Instruments Co., Ltd., Hitachinaka City, Ibaraki-ken, Japan. The cut-off values of CHD patients for tertiles distribution of each of the metabolic parameters were TC (mmol/L): I <4.83, II 4.83-6.35, III >6.35; TG (mmol/L): I <1.37, II 1.37-1.64, III >1.64; HDL-C (mmol/L): I <1.02, II 1.02-1.61, III >1.61; LDL-C (mmol/L): I <2.40, II 2.40-4.32, III >4.32; GLU (mmol/L): I <5.05, II 5.05-5.83, III >5.83; UA (µmol/L): I <342.61, II 342.61-446.84, III >446.84. The anthropometric variables of CHD patients, including blood pressure (SBP and DBP), body weight and height were measured with standard techniques. The BMI was calculated as body weight (kg) divided by the square of height (m). The cut-off values of CHD patients for tertiles distribution of each of the anthropometric variables were SBP (mmHg): I <148, II 148-182, III >182; DBP (mmHg): I <96, II 96-102, III >102; BMI (kg/m2): I <23.04, II 23.04-27.37, III >27.37; age (year): I <49, II 49-66, III >66.
Statistical analysis
The data were analyzed by using the Statistical Package for Social Science version 16.0 (SPSS Inc., Chicago, IL, USA). Categorical variables were expressed as percentage and compared by χ2-test. The correlation coefficients of Hcy with each of the metabolic parameters and anthropometric variables were calculated by Pearson’s analysis. The odds ratio (OR) and its 95% confidence intervals (95% CI) of HHcy with each of the metabolic parameters and anthropometric variables as well as life style habits (quantitative data expressed as tertiles, qualitative variables expressed as yes or no) were calculated by univariate logistic regression analysis. To select the variables independently associated with HHcy, variables with a p<0.25 in the univariate logistic regression analysis, including CHD category, folic acid, vitamin B12, age, BMI, gender, and smoking were retained and evaluated for further multivariate logistic regression analysis model (method: Stepwise Forward Wald). A p-value <0.05 was considered as significant.
Results
Principal characteristics of 292 CHD patients aged 36-85 years according to serum Hcy levels
The principal characteristics of 292 CHD patients aged 36-85 years according to serum Hcy levels are reported in Table 1. Compared with patients with normal-low Hcy concentrations, patients with HHcy were characterized by smoking, diabetes mellitus, hypercholesterolemia, hypertriglyceridemia, and risk factors ≥3 (p<0.05). There were no significant differences in the ratio of older ages, males, alcohol drinkers, hypertension, overweight or obesity between HHcy and normal-low Hcy groups.
Principal characteristics of 292 coronary heart disease patients aged 36-85 years according to serum homocysteine (Hcy) levels.
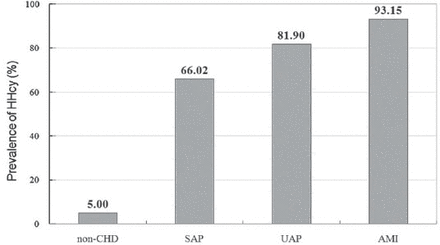
Comparison of prevalence of HHcy between CHD patients and non-CHD controls
Approximately four-fifths of CHD patients (79.1%) had a prevalence of HHcy, while only 5% of non-CHD patients had elevated serum Hcy concentrations. The prevalence of HHcy had a significant trend toward increase from non-CHD to AMI (χ2=184.51, p<0.001). The prevalence of HHcy in CHD patients and non-CHD controls is shown in Figure 1.
Prevalence of hyperhomocysteinemia (HHcy) in 292 coronary heart disease (CHD) patients with different categories and 100 non-CHD controls. SAP - stable angina pectoris, UAP - unstable angina pectoris, AMI - acute myocardial infarction
Associations between HHcy and each of the variables in CHD patients by univariate logistic regression analysis
The univariate logistic regression analysis revealed that HHcy was significantly associated with the severity of CHD (AMI versus SAP, OR: 18.57, 95% CI: 4.29-80.38, p<0.001; UAP versus SAP, OR: 1.99, 95% CI: 1.09-3.64, p=0.026), gender (male versus female, OR: 2.14, 95% CI: 1.20-3.79, p=0.010), smoking (yes versus no, OR: 2.89, 95% CI: 1.54-5.40, p=0.001), and was not significantly associated with alcohol intake (yes versus no, OR: 0.79, 95% CI: 0.44-1.44, p=0.442). The associations between HHcy and each of the quantitative variables in CHD patients are reported in Table 2.
Univariate logistic regression analysis of the association between hyperhomocysteinemia with each of the metabolic parameters and anthropometric variables in coronary heart disease patients.
Independent association of HHcy with variables in CHD patients by multivariate logistic regression analysis
After adjusting for confounding factors, the results of multivariate logistic regression analysis are shown in Table 3. The severity of CHD (AMI versus SAP), folic acid (III versus I tertile), vitamin B12 (III versus I tertile) and smoking (yes versus no) were independently associated with HHcy in CHD patients (p<0.05).
Independent association of hyperhomocysteinemia (HHcy) with the variables in coronary heart disease (CHD) patients by multivariate logistic regression analysis (stepwise forward Wald).
Correlations of Hcy with each of the metabolic parameters and anthropometric variables in CHD patients by Pearson’s analysis
The correlations of serum Hcy with each of the metabolic parameters and anthropometric variables in CHD patients are shown in Table 4. The Hcy was negatively correlated with folic acid, vitamin B12, and HDL-C levels, and positively correlated with TC, LDL-C, age, SBP, DBP, and BMI. On the contrary, Hcy was not significantly correlated with TG, GLU, and UA.
Pearson’s correlation coefficients of serum homocysteine (Hcy) with each of the metabolic parameters and anthropometric variables in coronary heart disease patients.
Comparison of the prevalence of low folic acid and vitamin B12 between the HHcy group and the normal-low Hcy group in CHD patients
More than half of the CHD patients (51.1%) with HHcy had low folic acid levels, 7 times higher than that of CHD patients (6.6%) with normal-low Hcy concentrations (χ2=39.33, p<0.001), and 42% of CHD patients with HHcy had low vitamin B12 levels, 5 times higher than that of CHD patients (8.2%) with normal-low Hcy concentrations (c2=24.25, p<0.001).
Discussion
The endothelium has a pivotal role in regulating blood flow, coagulation reactions, platelet activation, leukocyte adhesion, and vascular muscle function.11 Endothelial dysfunction, usually defined as an impairment of endothelium-dependent relaxation of blood vessels, plays an important role in the process of the development of arteriosclerosis, which is considered to be the initiating factor and the key point of cardiovascular disease.12 A major mechanism of endothelial dysfunction appears to be related to decreased bioavailability of endothelium-derived nitric oxide (NO). Nitric oxide is a major mediator of endothelium-dependent relaxation that is a potent vasodilator activating soluble guanylyl cyclase in vascular muscle, resulting in accumulation of cyclic guanosine monophosphate (cGMP) and relaxation, which is produced by endothelial NO synthase (eNOS) in response to physiological stimuli such as acetylcholine, thrombin, bradykinin, or shear stress, and is mainly reduced due to the uncoupling of eNOS by different mechanisms involving oxidative stress and inflammation.13 The Hcy is a sulfur-containing amino acid that functions as a key intermediate in methionine metabolism of demethylation. The HHcy, clinically defined as greater than 15 µmol/L of serum Hcy concentration, increases levels of asymmetric dimethylarginine (ADMA),14 which may cause uncoupling of eNOS, resulting in decreased production of NO and increased production of superoxide. The oxidative reaction of superoxide with NO generates peroxynitrite and decreases NO bioavailability, leading to endothelial dysfunction. Accumulation of S-adenosylhomocysteine, the Hcy precursor and a potent methyltransferase inhibitor, mediates the vascular complications associated with HHcy. Protein arginine methylation is a crucial post-translational modification and generates mono methylarginine and ADMA residues.15 Studies have confirmed that increased levels of the eNOS inhibitor ADMA have been associated with HHcy, and may contribute, at least in part, to the Hcy-induced endothelial dysfunction, and the negative vascular effects of HHcy have an ADMA-independent etiology.16 Fabian et al17 investigated the cardiovascular risk factors of Hcy and ADMA in elderly people, and reconfirmed that endothelial dysfunction of NO-mediated relaxation in patients with HHcy is due to impaired bioavailability of NO caused by Hcy-induced accumulation of ADMA.
A large number of studies have shown that HHcy was an independent risk factor for cardiovascular disease and elevated serum Hcy level was associated with CHD events.18-21 Esteghamati et al’s22 prospective study of 2 sub-cohorts over 8.5 years follow-up revealed that Hcy was correlated with metabolic syndrome components. Both HHcy and metabolic syndrome interacted as risk factors for CHD. Verdoia et al23 reported that elevated Hcy levels were associated with older age, male gender, hypertension, diabetes mellitus, and so on. In the present study, compared to patients with normal-low Hcy concentrations, patients with HHcy were characterized by smoking, diabetes mellitus, hypercholesterolemia, and hypertriglyceridemia (p<0.05).
The prevalence of HHcy in CHD patients was 79.1%, while only 5% of non-CHD patients had elevated serum Hcy concentrations. The prevalence of HHcy significantly increased from 5% in the non-CHD controlled group to 66% in the SAP group, to 81.9% in the UAP group, and to 93.15% in the AMI group (p<0.001). These findings confirm that HHcy is associated with the severity of CHD, as well as some traditional cardiovascular risk factors. A meta-analysis found that a 25% elevation (approximately 3 µmol/L) in serum total Hcy was associated with a 10% higher risk of cardiovascular events, and a 20% higher risk of stroke after adjustment for other known risk factors.24 Another systematic review and meta-analysis of 26 articles found that each elevation of 5 µmol/L in Hcy level increases the risk of CHD events by approximately 20%, independently of traditional CHD risk factors.25 More recently, several studies have investigated the association between Hcy and CHD events. Veeranna et al26 performed the Multi-Ethnic Study of Atherosclerosis and National Health and Nutrition Examination Survey III datasets found that Hcy more than 15 µmol/L significantly predicted CHD events (adjusted hazard ratio: 2.22, 95% CI: 1.20-4.09, p=0.01). Drewes et al27 in the PROspective Study of Pravastatin in the Elderly at Risk, a double-blind, randomized, placebo-controlled trial with 3.2 years follow-up, concluded that patients with a high Hcy level had a 1.8 higher risk (95% CI: 1.2-2.5, p=0.001) of fatal and nonfatal CHD than those with a low Hcy level.
In present study, univariate logistic regression analysis revealed that HHcy was significantly associated with the severity of CHD (AMI versus SAP, UAP versus SAP), gender (male versus female), smoking (yes versus no), folic acid (III versus I tertile), vitamin B12 (III versus I tertile), age (III versus I tertile), and BMI (III versus I tertile). After adjusting for confounding factors of gender, age, and BMI, multivariate logistic regression analysis showed that the severity of CHD (AMI versus SAP), folic acid (III versus I tertile), vitamin B12 (III versus I tertile), and smoking (yes versus no) are independently associated with HHcy (p<0.05). Among them, HHcy with the severity of CHD (AMI versus SAP, OR: 6.38, 95% CI: 1.18-34.46, p=0.031) showed the highest odds ratio. The present study provides valuable evidence that HHcy is independently associated with the severity of CHD. Hyperhomocysteinemia had extensive effects, damaging the vascular endothelial cells as the mercapto group of Hcy was highly reactive through oxidative stress, endoplasmic reticulum stress, promoted artery calcification, elevated serum levels of ADMA, involved in inflammation and autoimmunity, and mediated hypomethylation multiple mechanisms, and so on.28 The study has shown that vascular endothelial cells as a major target for the formation of atherosclerotic lesions, oxidative stress, and endoplasmic reticulum stress promote atherogenesis and thrombogenesis by producing superoxide and peroxynitrite,29 enhancing the inflammatory reaction, mediating expression and secretion of monocyte chemoattractant protein-1 and interleukin-8 in human monocytes, inducing programmed cell death, and endothelial dysfunction. The ADMA that accumulates in HHcy results in uncoupling of eNOS, reducing levels of NO and impairing NO bioavailability.30 Further products of peroxynitrite would induce endothelial dysfunction and thrombogenesis. Moreover, peroxynitrite in turn may cause further uncoupling of NOS. Thus forming a vicious cycle, increasing the occurrence and development of CHD.31
Folic acid and vitamin B12 play an important role in regulating the metabolic process of Hcy.32,33 A meta-analysis34 that included 14 randomized controlled trials with 54913 participants found a reduction in overall stroke events resulting from reduction in Hcy levels following B vitamin supplementation. Cheng et al’s35 investigation has also demonstrated that supplements containing folic acid, vitamin B6, and vitamin B12 in middle-aged and elderly patients with HHcy could reduce serum Hcy levels. Our study revealed that Hcy was negatively correlated with folic acid (r=-0.67, p<0.001) and vitamin B12 (r=-0.56, p<0.001). Of the CHD patients with HHcy, 51.1% had low folic acid levels, 7 times higher than that of CHD patients (6.6%) with normal-low Hcy concentrations (p<0.001). Similarly, 42% of CHD patients with HHcy had low vitamin B12 levels, 5 times higher than that of CHD patients (8.2%) with normal-low Hcy concentrations (p<0.001). Our results confirm that serum folic acid and vitamin B12 influence Hcy metabolism as a cosubstrate and a cofactor, and low levels of folic acid and vitamin B12 are significantly correlated with HHcy. Folic acid and vitamin B12 deficiency results in elevated levels of Hcy.36,37 Approximately 50% of Hcy is metabolized via transsulfuration to cystathionine, and the remaining 50% of Hcy is re-synthetized to methionine by re-methylation with the enzyme methionine synthase. Hcy re-methylation requires vitamin B12 and 5,10-methyl-tetra-hydrofolic acid (MTHF), which is generated by 5,10-MTHF reductase.38 Vitamin B12 is an agon of methionine synthetase that catalyzes this reaction, and participate in the transfusion of methyl.39 Folic acid deficiency will prevent remethylation of Hcy because of raw material deficiency. Moreover, folic acid deficiency will also influence the production of MTHF by affecting the activity of methylene tetrahydrofolate reductase.40,41 Vitamin B12 deficiency will also result in HHcy because re-methylating Hcy to methionine is reduced, cannot transfer, which influences the production of methionine and regeneration of tetrahydro-folic acid.42
Study limitations
Since the present study was just a single site retrospective investigation, a multicenter study with prospective investigation is necessary to further observe the association between Hcy levels and the severity of CHD.
In conclusion, the present study provides valuable evidence that HHcy is independently associated with the severity of CHD. Folic acid and vitamin B12 influence Hcy metabolism as cosubstrate and cofactor, and low levels of folic acid and vitamin B12 are significantly correlated with HHcy.
Acknowledgment
The authors gratefully acknowledge the staff in the Department of Clinical Laboratory and Department of Cardiovascular at the Sichuan Academy of Medical Sciences & Sichuan Provincial People’s Hospital, Chengdu, China for their support and guidance.
Footnotes
Disclosure. Authors have no conflict of interests, and the work was not supported or funded by any drug company. This research was supported by the National High Technology Research and Development Program of China (863 Program) (No. 2011AA 02A 111), and Sichuan Provincial Science and Technology Department Research Foundation of China (No. 2013FZ0080), Chengdu, China.
- Received March 8, 2015.
- Accepted May 18, 2015.
- Copyright: © Saudi Medical Journal
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.