Abstract
Objectives: To explore the antibacterial activity of thymoquinone (TQ), a quinone extracted from Nigella sativa.
Methods: This study was conducted from May 2019 to March 2020 at the Department of Clinical Laboratory Sciences, College of Applied Medical Sciences, King Khalid University, Abha, Saudi Arabia. The antimicrobial activity, minimum inhibitory concentration (MIC), and minimum bactericidal concentration (MBC) of TQ were determined using an agar well diffusion method and broth microdilution assays, and the synergistic effect was evaluated using antibiotics in parallel. The disruptive effect of TQ on bacterial cell membranes was determined using scanning electron microscopy. The antivirulence properties of TQ, which include adherence and biofilm formation, were also investigated using adherence and biofilm formation assays, respectively.
Results: Thymoquinone demonstrated bactericidal efficacy against 4/14 bacterial strains, with MIC range of 1.04-8.3 µg/mL and and MBC range of 10.41–66.66 µg/mL. Thymoquinone showed synergism against Klebsiella pneumoniae, Staphylococcus epidermidis (American Type Culture Collection 12228), Staphylococcus aureus, and Staphylococcus epidermidis in combination with the tested antibiotics. Thymoquinone inhibited bacterial adhesion by 39%-54%, 48%-68%, and 61%-81% at 0.5 × MIC, 1 × MIC, and 2 × MIC, respectively. The tested bacterial strains significantly inhibited biofilm formation after treatment with various concentrations of TQ for 24 and 48 hours.
Conclusion: The combinatory effect of TQ with antimicrobials should be considered when developing new antimicrobial therapy regimens to overcome multidrug-resistant.
Antibiotic resistance has become a major health issue over the past decade due to the rapid growth of multidrug-resistant (MDR) microbes.1 Antimicrobial drugs are effective in bacterial infection control. However, microbes have the ability to develop resistance to several active antimicrobials.2 Due to the emergence of multidrug resistance and the insufficiency of new antimicrobials, novel and innovative approaches to combat MDR bacteria are crucial.3,4 However, bacteria efficiently develop resistance to antimicrobials soon after they have been introduced, and the majority of antibiotics have side effects.5 Therefore, searches for new drugs or combinations with less toxicity and uncompromised efficacy against resistant strains are continually evolving.6,7 Nonetheless, the issue of bacterial resistance to antibiotics was resolved by the uncovering of new antibiotics, like glycopeptides, aminoglycosides, and macrolides, as well as by chemically modifying existing medicines.1
A potential strategy to minimize microbial resistance is the administration of antimicrobials in combination with naturally available compounds.1,8 Phytochemicals synergistically enhance the effect of traditional antimicrobials.9 Thymoquinone (TQ; 2-isopropyl-5-methyl-1,4-benzoquinone) is an essential active compound of Nigella sativa (N. sativa) L. seeds (black seed), which have been traditionally used for decades in the Middle East, Northern Africa, and India as a natural remedy for many contagious diseases such as bronchitis, pulmonary infection, cough, influenza, and fever.10-11 Thymoquinone exerts various pharmacological effects and has antiinflammatory, anticancer, antidiabetic, antiasthmatic, hypolipidemic, antihypertensive, nephroprotective, and antimicrobial activities.12-16
The antibacterial effects of TQ and other N. sativa extracts have been determined against Staphylococcus aureus (S. aureus), Pseudomonas aeruginosa (P. aeruginosa), Listeria monocytogenes, and Escherichia coli (E. coli).17-19 Thymoquinone exerts inhibitory effects against gram-negative and gram-positive bacteria.20 A diethyl ether extract of N. sativa showed synergy with streptomycin and gentamicin and an additive effect with erythromycin, streptomycin, doxycycline, tobramycin, nalidixic acid, chloramphenicol, ampicillin, co-trimoxazole, and lincomycin.21 Furthermore, both antibiotic-sensitive and MDR gram-positive and gram-negative bacterial isolates are susceptible to N. sativa extracts.22 This study aimed to further elucidate the potential effect of TQ against clinically significant bacteria by investigating the antibacterial effect of TQ alone and in combination with different antibiotics against drug-resistant isolates.
Methods
This is an experimental study which was conducted from May 2019 to March 2020 in the Department of Clinical Laboratory Sciences, College of Applied Medical Sciences, King Khalid University, Abha, Saudi Arabia.
Organisms and chemicals
The antibacterial effect of TQ was examined using pathogenic strains, including the gram-negative bacteria P. aeruginosa (American Type Culture Collection [ATCC] 27853), E. coli (ATCC 25922), E. coli, Salmonella typhi, Pseudomonas sp., Klebsiella pneumoniae (K. pneumoniae), and Shigella sp.), and the gram-positive bacteria Enterococcus faecalis (ATCC 29212), S. epidermidis (ATCC 12228), S. aureus, S. saprophyticus, S. epidermidis, Streptococcus pyogenes, and Mycobacterium smegmatis. Gentamycin, ofloxacin, tetracycline, penicillin, chloramphenicol, nalidixic acid, and TQ were purchased from Sigma (Sigma-Aldrich, Switzerland).
Antimicrobial susceptibility assays
The antimicrobial activity of the compounds was evaluated using an agar well diffusion method. The bacterial culture was inoculated in lysogeny broth and incubated at 37°C for 3 h (hours). The turbidity of the inoculum was consequently adjusted using phosphate-buffered saline (PBS) to be equivalent to 0.5 McFarland’s standard. Then, 20 µL of TQ (50 µg/mL) was transferred into each well of the Petri dishes, which were then incubated aerobically at 37°C for 24 hours (h). The width of the zone of inhibition of bacterial growth surrounding each well was calculated in millimeters, as previously described.23
Determination of minimum inhibitory concentration (MIC)
The MICs of TQ against the bacterial strains were determined by broth microdilution assays using TQ concentrations of 0.024-50 µg/mL. Two-fold dilutions of TQ (100 µL) were pipetted into the wells of a sterile flat-bottomed 96-well plate for each strain under test. The initial inoculum concentration for each strain was 1.5 × 105 colony-forming units/mL. The control wells contained the bacterial inoculum only. The plate was incubated aerobically 37°C for 24 h. The lowest TQ concentration that exhibited neither turbidity nor visible bacterial growth after the 24-h incubation was considered the MIC.
Determination of minimum bactericidal concentration (MBC)
After the 24-h incubation, 100 µL of inoculum from each well of the broth microdilution assay plate was sub-cultured on Mueller–Hinton agar (MHA) plates, which were then incubated for 24 h. The minimum concentration of TQ that resulted in no bacterial growth was considered the MBC.
Synergistic antimicrobial assays
The synergistic antimicrobial activity of antibiotics (gentamycin, ofloxacin, tetracycline, penicillin, chloramphenicol, and nalidixic acid) in combination with TQ was investigated. Bacterial strains at a turbidity of 0.5 McFarland’s standard were spread on MHA plates. The specific antibiotic discs, which had been kept aerobically at 37°C for 24 h, were separately soaked in 5 µL of TQ (at the MBC value) and placed on the inoculated MHA plates. Then, the plates were incubated aerobically at 37°C for 24 h. The inhibition zones formed by TQ in combination with the selected antibiotics after overnight incubation were calculated, as previously described.24
Scanning electron microscopy (SEM)
Scanning electron microscopy was used to investigate the effect of TQ on cell morphology.25 Scanning electron microscopy sample preparation was initially carried out in centrifuge tubes. Then, the bacterial specimens were instantly fixed in 2.5% (wt/vol) phosphate-buffered glutaraldehyde (pH 7.4) at 4°C for 4 h, followed by post-fixation in 1% phosphate-buffered osmium tetroxide (pH 7.4) for 1 h. After washing and dehydration in ascending grades of ethanol, critical-point drying was performed using an EMitech K850 critical-point dryer. The samples were mounted on aluminum mounts with silver glue and then sputtered with a gold coat using a BOC Edwards Scancoat sputter coater. The specimens were examined using a JSM-6390LV scanning electron microscope (Jeol Ltd., Japan).26
Adherence assay
Bacterial adherence was assessed as described elsewhere.27 Briefly, 100 µL of the bacterial cells under test (optical density at 610 nm [OD610 nm]=0.01) grown in Roswell Park Memorial Institute (RPMI),New York, United States in America (USA) 1640 media and buffered with 0.165 M morpholinepropanesulphonic acid (MOPS) (Sigma-Aldrich, MO, USA) at pH 7.0 were loaded into all wells of a 96-well plate. Then, the bacterial cells were treated with different concentrations of TQ (0.5 × MIC, 1 × MIC, and 2 × MIC) and incubated for 6 h at 37°C. Untreated bacterial cells were used in each set of experiments as negative controls. Following incubation, the media were discarded, and each well was rinsed twice with 200 µL PBS to eliminate the non-adherent bacterial cells. Then, 100 µL of Alamar blue at an absolute concentration of 5% in RPMI 1640 media was loaded into each well, followed by incubation at 37°C for 6 h. The amount of fluorescence was measured at 555 and 585 nm for excitation and emission, respectively, using a Synergy HT microplate reader (BioTek Instruments, VT, USA).
Biofilm formation assay
Biofilm production was assessed by the inoculating the tested bacterial cells suspensions (OD610 nm=0.01) grown in RPMI 1640 media buffered with 0.165 M MOPS (pH 7.0) into the wells of a sterile flat-bottomed 96-well plate, and the plate was incubated for 6 h at 37°C.28 Thereafter, the culture medium was carefully removed without disturbing the formation of biofilm. Then, different TQ concentrations (0.5 × MIC, 1 × MIC, and 2 × MIC) were prepared in fresh RPMI 1640 medium and loaded into the wells. Untreated bacterial cells were used as negative controls in each set of experiments. The 96-well plate was further incubated at 37°C, and the effect of TQ on biofilm formation was assessed after 24 h and 48 h, as previously described.29
Statistical analysis
Each investigation was conducted in triplicate, and the results were presented as means ± standard deviation (SD). GraphPad Prism software v6.0 (GraphPad, CA, USA) was used to perform the statistical analyses. One-way analysis of variance with Dunnett’s multiple comparison test was used to compare the mean between different groups with that of the control. P-values <0.05 were considered statistically significant.
Results
Antibacterial activity of TQ
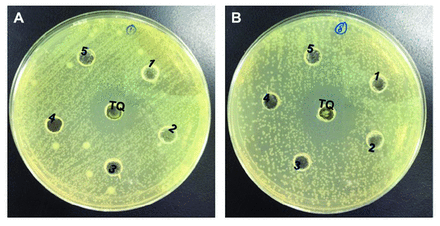
Thymoquinone exerted a bactericidal effect on 4/14 strains, with MICs of 1.04-8.3 µg/mL and MBCs of 10.41-66.66 µg/mL (Table 1 & Figure 1). This effect was almost indistinguishable from the tested antibiotics (ofloxacin, gentamycin, tetracycline, penicillin, chloramphenicol, and nalidixic acid). The gram-negative bacteria E. coli ATCC 25922, P. aeruginosa ATCC 27853, Pseudomonas sp., Salmonella typhi, Shigella sp., and E. coli were resistant to TQ. However, for gram-positive bacteria, only Enterococcus faecalis ATCC 29212, M. smegmatis, S. saprophyticus, and S. pyogenes appeared resistant to TQ.
- Antibacterial activity of TQ. A) Antibacterial acitivity of thymoquinone (TQ) against Klebsiella pneumoniae. B) Antibacterial acitivity of TQ against Staphylococcus epidermidis (ATCC 1228).
- Antibacterial activity of thymoquinone against pathogenic bacterial strains.
Synergism action
For gram-positive bacteria, TQ showed synergism in combination with gentamicin and penicillin against S. epidermidis ATCC 12228; with ofloxacin, tetracycline, and chloramphenicol against S aureus; and with ofloxacin and penicillin against S. epidermidis. For gram-negative bacteria, TQ showed synergism with gentamicin, ofloxacin, penicillin, and nalidixic acid against K. pneumoniae (Table 2).
- Aynergistic antimicrobial activity of thymoquinone (TQ) with different antimicrobial agents.
Antagonism action
For gram-positive bacteria, TQ showed antagonism against S. epidermidis ATCC 12228 when combined with ofloxacin, tetracycline, and chloramphenicol; S. aureus with penicillin; and S. epidermidis with tetracycline and chloramphenicol. However, for gram-negative bacteria, TQ antagonism was not detected against K. pneumonia in combination with any of the tested antibiotics (Table 2).
Additive action
For gram-positive bacteria, TQ showed additive action with nalidixic acid against S. epidermidis ATCC 12228 and with gentamicin and nalidixic acid against S. aureus and S. epidermidis. For gram-negative bacteria, TQ showed additive action with tetracycline and chloramphenicol against K. pneumoniae. Most of the results are same during each trial and there were no any significant difference observed with α=0.05.
Disruption of bacterial cells visualized by SEM
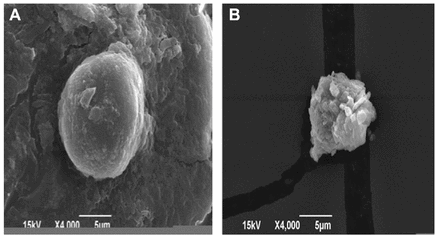
Scanning electron microscopy was used to observed changes in the structural morphology of S. epidermidis in response to TQ (Figure 2). In the absence of TQ (control), the cells showed characteristic normal morphology (Figure 2A). Unlike the control, the cells that were treated with TQ had damaged cell membranes, showing that TQ treatment reduced the synthesis of extracellular polysaccharides (Figure 2B).
- Effect of thymoquinone (TQ) on cell morphology of Staphylococcus epidermidis. Changes in the cellular morphology were observed by scanning electron microscopy. A) Control cell B) shows bacterial cell treated with TQ.
Thymoquinone inhibits bacterial adhesion
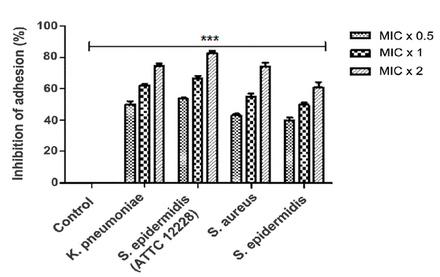
Adhesion assays were performed using Alamar blue dye to assess the effect of TQ on bacterial adherence. The bacterial cells showed inconsistent inhibition of adherence following exposure to various TQ concentrations, and this was concentration-dependent. For K. pneumoniae, S. epidermidis (ATCC 12228), S. aureus, and S. epidermidis, TQ inhibited bacterial adhesion by 39%-54% at 0.5 × MIC, 48%-68% at 1 × MIC, and 61%-81% at 2 × MIC (Figure 3), indicating that TQ was extremely efficient in diminishing adhesion of the tested bacteria, even at subinhibitory concentrations.
- Thymoquinone (TQ) inhibits bacterial adhesion. Alamar Blue based polystyrene adhesion assay was used to evaluate the effect of TQ on Klebsiella pneumoniae, Staphylococcus epidermidis (ATCC 12228), Staphylococcus aureus and Staphylococcus epidermidis adherence. All the tested bacterial strains were exposed to MIC x 0.5, MIC x 1 and MIC x 2 values of TQ for 6 h at 37°C. Control bars indicate all untreated bacterial strains, accepted as 0% inhibition. Results are presented from three independent experiments using means ± SD ***p<0.0001.
Thymoquinone inhibits bacterial biofilm formation
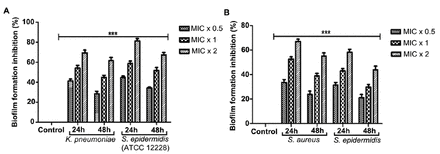
TQ treatment at 0.5 × MIC, 1 × MIC, and 2 × MIC significantly inhibited biofilm formation in K. pneumoniae, S. epidermidis (ATCC 12228), S. aureus, and S. epidermidis by 31%-45%, 41%-59%, and 58%-81%, respectively, after 24 h and by 21%-34%, 30%-51%, and 44%-67%, respectively, after 48 h (Figure 4). The rate of biofilm formation inhibition by TQ was concentration- and time-dependent.
- Thymoquinone (TQ) reduces biofilm formation. A) Klebsiella pneumoniae and Staphylococcus epidermidis (ATCC 12228), B) Staphylococcus aureus and Staphylococcus epidermidis were incubated with MIC x 0.5, MIC x 1 and MIC x 2 values of TQ under biofilm growing conditions for 24 and 48 h. Control bars indicate all untreated bacterial strains, accepted as 0% inhibition. Results are presented from 3 independent experiments using means±SD ***p<0.0001.
Discussion
Thymoquinone is one of the most bioactive components of N. sativa and has diverse beneficial properties. Regarding antimicrobial effects, TQ has a broad-spectrum efficacy encompassing gram-negative and gram-positive bacteria.27 Thymoquinone also exhibits a synergistic effect with gentamicin and streptomycin and an additive effect with spectinomycin, doxycycline, tobramycin, erythromycin, chloramphenicol, ampicillin, lincomycin, nalidixic acid, and a combination of sulfamethoxazole and trimethoprim.21,27 In our study, TQ exhibited synergism in combination with gentamicin (p=0.254) and penicillin (p=0.471) against gram-positive S. epidermidis (ATCC 12228). Other studies have reported a similar effect against S. epidermidis in combination with ofloxacin and penicillin. Furthermore, S. epidermidis strains were reported as resistant to more than one antibiotic, and S. epidermidis isolates were also more sensitive to ofloxacin and gentamycin. Combined with antibiotics, these natural extracts showed significant synergy, and the maximum synergistic effect was detected with cephalexin and erythromycin.30-32
Methicillin-resistant S. aureus is the most common pathogen encountered in clinical and laboratory settings.27 Previous studies have suggested that TQ in combination with antibiotics, such as cephalexin, ampicillin, tetracycline, gentamicin, and chloramphenicol, may show synergistic activity against S. aureus.23 Our observations agreed with the literature, as TQ showed a synergistic effect with ofloxacin, tetracycline, and chloramphenicol against S. aureus.7,27 Furthermore, our observations are supported by a report of TQ increasing the anti-staphylococcal effect of penicillin, tetracycline, and oxacillin against resistant bacterial strains.33
The MIC values that we observed for TQ against K. pneumoniae were much higher than those in wild-type E. coli. Therefore, it is evident that K. pneumoniae is highly resistant to several antimicrobial agents. Multidrug-resistant efflux pumps are the most important mechanisms through which bacteria can resist antibiotics, most notably tetracyclines.33 This pump expels multiple drugs and could play a role in multidrug resistance.34 To the best of our knowledge, there is a lack of literature on TQ synergistic studies with antibiotics against K. pneumoniae. In our study, TQ was synergistic with gentamicin (p=0.458), ofloxacin (p=0.638), penicillin (p=0.485), and nalidixic acid (p≤0.001) against gram-negative K. pneumoniae. Thymoquinone has been reported to exert antibacterial activity against both gram-positive and gram-negative bacteria. Furthermore, MDR clinical strains of Shigella flexneri, S. typhimurium, S. aureus, and S. enteritidis were reported with TQ sensitivity similar to that of non-MDR strains, and the susceptibility of resistant bacterial isolates to TQ has been reported.35 Clinical isolates of S. aureus are often resistant to nearly all of the frequently used antimicrobials, including macrolides, aminoglycosides, chloramphenicol, fluoroquinolones, and tetracycline.36 Our study demonstrated that TQ exerts synergistic lethal effects against both gram-negative and gram-positive bacteria when combined with antibiotics. Therefore, TQ could be used as an antibacterial drug.
Efflux pumps are a bacterial defense mechanism that eliminates antibiotics from the cell. The action of the efflux pumps often predisposes the progression of microorganisms toward being highly resistant to antimicrobials.37,38 Some investigations have demonstrated the vital function of phytochemistry to explore the effectiveness of efflux pump inhibitors, particularly against gram-positive bacteria such as S. epidermidis and S. aureus.30 It was also reported that TQ could inhibit efflux pumps, consequently enhancing the concentration of 4’,6-diamidino-2-phenylindole in the cells.38 Hence, we hypothesize that this potential mechanism could also play a role in the anti-staphylococcal activity of TQ-tetracycline in combination with antibiotics, which is consistent with our study results. In contrast, -lactams are generally responsible for staphylococcal resistance by producing modified penicillin-binding proteins (PBPs) with decreased binding properties to antimicrobial agents.39 Therefore, it has been suggested that TQ may play a role in inhibiting PBPs responsible for staphylococcal resistance.34 Previous findings demonstrated that TQ could be an effective compound in combination with other antimicrobials against many bacterial strains, especially Staphylococcus sp. However, further investigations are necessary to examine their practical application.40
The capability of bacteria to adhere and form biofilm is responsible for their virulence. Our study revealed that TQ inhibited bacterial adhesion and biofilm formation. Adhesion properties are directly related to biofilm formation and lead to plaque formation. Bacterial cells have specialized proteins (adhesins) that encode host cell surface glycoproteins, enabling bacteria to adhere to host cells.41 We found that TQ significantly inhibited adherence by 39%-81% in a dose-dependent manner in K. pneumoniae, S. epidermidis (ATCC 12228), S. aureus, and S. epidermidis, and adherence to host tissue lead to biofilm formation in these strains. Biofilm is a vital virulence factor in periodontal bacteria. It enhances resistance to most conventional antibacterial drugs.42 Furthermore, there are numerous reports that biofilms are more resistant than planktonic cells to existing antimicrobials.43 In our study, TQ treatment significantly suppressed biofilm formation in a concentration-dependent manner by 31%-81% for 24 h and 21%-67% for 48 h. Our findings support the assumption that TQ targets membrane adhesion proteins to inhibit adherence to host cells, subsequently inhibiting biofilm formation.
In conclusion, our study demonstrates that TQ can enhance the antibacterial activity of some antimicrobials. As demonstrated in Table 1, TQ was found more efficient anti-bacterial activities particularly against gram-positive bacteria. Although our findings provide new insights into possible mechanistic scenarios associated with the combinatory effect of TQ with antimicrobials. Further investigations are necessary to facilitate the development of novel therapeutic strategies using TQ against resistant microbes.
Acknowledgment
The authors are thankful to the Deanship of Scientific Research, King Khalid University, Abha, Kingdom of Saudi Arabia, for financially supporting this work through the General Research Project under (grant number G.R.P-3-40). The authors also thank the Deanship of Scientific Research and Researcher’s Services & Support Unit, King Saud University, Riyadh, Kingdom of Saudi Arabia for their technical support. The authors also acknowledge Enago (www.enago.com) for English language editing.
Footnotes
Disclosure. This study was funded by the Deanship of Scientific Research, King Khalid University, Abha, Kingdom of Saudi Arabia (Grant Number: G.R.P-3-40).
- Received November 14, 2020.
- Accepted January 14, 2021.
- Copyright: © Saudi Medical Journal
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial License (CC BY-NC), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.