Abstract
Objectives: To compare the efficacy of prophylactic ondansetron and tropisetron for postoperative nausea and vomiting (PONV).
Methods: A literature search was performed to identify studies that compare the efficiency of ondansetron with that of tropisetron in preventing PONV. Only randomized controlled trials updated to January, 2021 were included.
Results: The final pooled analysis included 14 studies totaling 1705 patients and indicated that ondansetron was 39% less effective than tropisetron in preventing postoperative vomiting with a higher incidence of dizziness. However, no significant difference was detected between ondansetron and tropisetron in PONV, postoperative nausea, antiemetic treatment, and headache.
Conclusions: Tropisetron is superior to ondansetron in preventing postoperative vomiting.
PROSPERO No: CRD42021237368
Postoperative nausea and vomiting (PONV) is a distressing side effect after anesthesia,1 because it may cause some adverse effects such as deprivation of body fluids, electrolyte imbalance, delayed recovery, aspiration pneumonia, and decreased satisfaction of patients’ after surgery.2
Prophylactic administration of 5-hydroxytryptamine-3 (5-HT3) receptor antagonists has been utilized as an effective method for preventing PONV. Comparative studies between different 5-HT3 antagonists for preventing PONV failed to show a clear advantage of a specific 5-HT3 antagonist.3
This meta-analysis was designed to determine the effect of two 5-HT3 receptor antagonists with different half-lives in preventing PONV, that is, the short-acting ondansetron versus the relatively long-acting tropisetron.
Methods
Two investigators (NW, RW) identified the eligible studies by searching PubMed, Web of Science, Cochrane Library, and Google Scholar, using “prevention,” “nausea,” “vomiting,” “ondansetron,” and “tropisetron” as search terms updated to January, 2021. Potential randomized controlled trials (RCTs) were identified by a systematic search of reference lists from related articles.
Inclusion criteria were: a RCT study; patients should have undergone operation; records of PONV-data; ondansetron or tropisetron administered prophylactically; and ondansetron and tropisetron comparison. On the other hand, none-english articles, animal studies, children studies, and published abstracts, meeting papers and letters were excluded.
The quality of the RCTs was separately evaluated by 2 investigators (JW, XS) utilizing the Cochrane Collaboration guidelines and Jadad improvement score.4,5 Studies with Jadad improvement score of less than 4 were excluded.
Two independent investigators (YC, RW) extracted relevant data from the included studies. The primary outcome was PONV, while additional outcomes included the requirement of antiemetic treatment and the related complications. Any disagreement was solved by a third investigator.
Statistical analysis
Statistical calculations were conducted using Revman 5.3 (Cochrane Collaboration). The outcome was displayed as odds ratio (OR) with 95% confidence interval (CI). I2 value was utilized to evaluate heterogeneity. If I2 ≤50%, a fixed-effect model was peformed. Funnel plot and Egger test were utilized to assess publication bias. Statistical significance was p<0.05.
Results
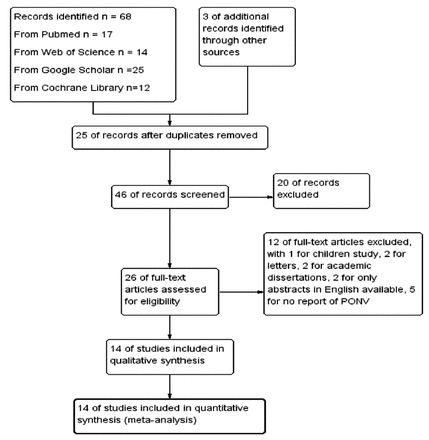
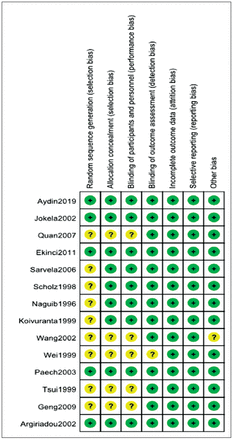
The literature search identified 68 articles initially. After reading the abstracts, 42 studies were excluded. Of the 26 remaining studies, 14 articles were included in this meta-analysis after reviewing the full manuscript (Figure 1).6-19 The characteristics of the 14 articles involving 1705 patients are summarized in Table 1. An overview of the risk of bias was shown in Figure 2.
- Flow diagram of literature search.
- Risk of bias summary.
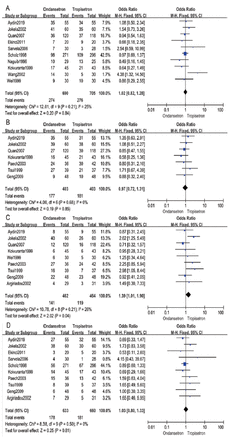
- Characteristics of the included studies.
As shown in Figure 3, 10 studies involving 1395 patients reported the incidence of PONV. The effect of ondansetron and tropisetron was equal in preventing PONV (OR: 1.02; 95% CI: 0.82-1.28; p=0.84; I2=25%) (Figure 3A).6-15
- Forest plot comparing between ondansetron and tropisetron: A) postoperative nausea and vomiting; B) postoperative nausea; C) postoperative vomiting; D) antiemetic treatment.
Postoperative nausea (PON) was assessed in 7 studies including 806 patients.6-8,16-18 Meanwhile, postoperative vomiting (POV) was reported in 9 studies including 926 patients.6-8,13,15-19 This meta-analysis indicated no difference in PON between ondansetron and tropisetron (OR: 0.97; 95% CI: 0.72–1.31; p=0.85; I2=0%) (Figure 3B). Ondansetron was 39% less effective than tropisetron in preventing POV (OR: 1.39; 95% CI: 1.01-1.90; p=0.04; I2=26%) (Figure 3C).
Antiemetic treatment
Antiemetic treatment was reported in 10 studies including 1293 patients.6,7,9-11,13,16-19 The difference in antiemetic treatment was not statistically significant between ondansetron and tropisetron (OR: 1.03; 95% CI: 0.80-1.33; p=0.81; I2 = 0%) (Figure 3D).
Complications
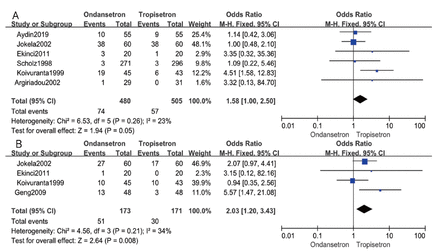
Headache was evaluated in 6 studies involving 985 patients.6,7,9,11,13,19 As displayed in Figure 4A, ondansetron compared to tropisetron tended to have higher incidence of headache; however, it wasn’t statistically significant (OR: 1.58; 95% CI: 1.00-2.50; p=0.05; I2=23%). On the other hand, dizziness was evaluated in 4 studies involving 344 patients.7,9,13,18 As shown in Figure 4B, ondansetron had 103% higher incidence of dizziness than that with tropisetron (OR: 2.03; 95% CI: 1.20-3.43; p=0.008; I2=34%).
- Forest plot of comparison of the side effects experienced by patients receiving ondansetron and tropisetron treatment: A) headache and B) dizziness.
Publication bias
The funnel plot of PONV was asymmetrical. However, Egger test did not reveal significant difference in PONV (p=0.501).
Discussion
Previous systematic review has shown that 5-HT3 receptor antagonists could prevent PONV.20 The mechanism may be that they can block vagal nerves which trigger the emetic reflex.21 Ondansetron is the original member of this class with a short elimination half-life, and its effect is confirmed in many studies of different patient populations. Tropisetron is also a potent 5-HT3 receptor antagonist with longer elimination half-life than that of ondansetron. It is produced by systematic methyl substitution of the serotonin molecules.22 It is still a matter of significant interest to compare the efficacy and side-effect profiles of the short-acting ondansetron and the relatively long-acting tropisetron prophylactically given to patients of both genders undergoing surgery.
The present meta-analysis indicated that tropisetron was more effective than ondansetron in preventing POV, and prophylactic ondansetron and tropisetron had similar incidence of PONV, incidence of PON, and antiemetic efficacy in adults.
We note a difference in the half-life time of ondansetron (T1/2 = 3.2 hours) and tropisetron (T1/2 = 7.3−8 hours), which is probably related to the lower percentage of patients who experienced POV in the tropisetron group.23 It indicates that prophylactic tropisetron can provide a more long-standing antiemetic coverage after surgery. However, tropisetron does not reduce the incidence of PONV and PON, and requirement for antiemetic treatment, as compared to that with ondansetron.
Furthermore, tropisetron causes fewer side effects than ondansetron. Compared with ondansetron, tropisetron can decrease the incidence of dizziness. Additionally, tropisetron tends to increase the incidence of headache; however, this difference was not statistically significant. If more RCTs are included and more patients are involved, tropisetron may be shown to be more effective. Nonetheless, we were able to demonstrate in this meta-analysis that tropisetron can more effectively prevent POV with a lower incidence of dizziness than ondansetron.
Several potential limitations associated with these results should be mentioned. First, 2 of the included RCTs had relatively small sample sizes, which might influence the credibility of the conclusion. Second, there were some clinical differences between the included studies: dosages and the administration routes of the study drugs in the included RCTs vary, which may affect the reliability of pooling effects. Finally, the optimal dosages of ondansetron and tropisetron were the remaining question, which need further attention.
In conclusion, tropisetron is superior to ondansetron in preventing POV. It is 39% more effective than ondansetron in preventing POV with a lower incidence of dizziness.
Acknowledgment
We would like to thank Editage (www.editage.cn) for English language editing.
Footnotes
Disclosure. Authors have no conflict of interests, and the work was not supported or funded by any drug company.
- Received March 18, 2021.
- Accepted May 31, 2021.
- Copyright: © Saudi Medical Journal
This is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.