Abstract
Objectives: To explore renal toxicity caused by sub-acute exposure of acrylamide and to study the protective effect of 5-Aminosalicylic acid (5-ASA) and Vitamin E (vit-E)on Acrylamide (ACR) induced renal toxicity.
Methods: This study was conducted at King Fahad Medical Research Centre, King Abdulaziz University, Jeddah, Kingdom of Saudi Arabia, between August and November 2015. A total of 49 adult Wistar rats (250 ± 20g) aged 60 days were kept in a controlled environment and used in the present study. The rats were divided into 7 groups (control, ACR alone, ACR+5-ASA, ACR+vit-E, ACR+ASA+vit-E, vit-E alone, and ASA alone). After 5 days of ACR oral gavage treatment, the rats were observed for 24 hours then killed. Histopathology for the kidney and lactate dehydrogenase assay were carried out.
Results: Acrylamide produced significant pathological changes in the kidney with acute tubular necrosis in the distal tubules that could be reversed by concomitant injection of rat with 5-ASA. Together with vitamin E, 5-ASA, showed maximum renal protection. No statistically significant difference was observed in either body weights or lactate dehydrogenase activity of ACR treated rats.
Conclusion: Acrylamide exposure leads to adverse clinical pathologies of renal tubules, which were reversed by a concomitant treatment with 5-ASA and vitamin-E
Acrylamide (ACR) possess an α-β unsaturated carbonyl compound having high chemical activity. Being a substrate for polyacrylamide synthesis it is extensively used in industrial processes.1 Thus, human absorption of polyacrylamides usually occurs by way of occupational exposure.2,3 Acrylamide has been classified as a potent human carcinogen by the International Agency for Research Cancer (IARC, 1995).4 Carcinogenicity of ACR has also been confirmed in rodents.5,6 In year 2002, Swedish National Food Administration reported the presence of high ACR concentration in carbohydrate rich foods, such as french fries and potato chips that were cooked under high temperature due to milliard reaction, which raised a worldwide concern.7 Since ACR is a water soluble compound, it has the capability of easily diffusing throughout the body system thus targeting all body tissues for ACR induced damage or carcinogenesis.8 In addition to its carcinogenic effects, ACR is also known as a neuro and reproductive toxicant in humans.9 However, little is known regarding the contribution of ACR in renal damage. Once absorbed, ACR is metabolized by 2 main pathways; glutathione conjugation or glycidamide epoxidation.10 It may either be conjugated by glutathione-S-transferase to N-acetyl cysteine or may react with cytochrome P2E1 (CYP2E1) to produce glycidamide. Toxicity of ACR is attributed to the fact that it can be bio-transformed to glycidamide, which is a highly reactive metabolite known to be more toxic towards proteins and DNA than ACR itself.11 Hence, the most pathogenic pathway of ACR is its transformation to glycidamide, a pathway, which is regulated by the enzyme cytochrome P450. In liver, the main enzyme involved in the production of glycidamide is CYP2E1 (member of cyt P450 family)12,13. Acrylamide metabolism by CYP2E1 causes release of free radicals (ROS), which initiates oxidative stress leading to an imbalance in production and destruction of ROS, hence causing lipid peroxidation.5,11 Other implications of oxidative stress are DNA and protein alterations.12,13 Many compounds are known to have excellent antioxidant and anti-inflammatory properties, 5- amino salicylic acid, being one among them.14,15 Since ACR exposure leads to oxidative stress, antioxidants such as vit-E and 5-ASA could be used as potential ameliorators of ACR toxicity. There is a lack of data, which could establish the direct effect of ACR exposure on renal toxicity. However, some studies show the association between dietary ACR intake and renal cell carcinoma.11 Also, Totani et al16 and Mansour et al17 have reported periglomerular oedema, with infiltration of inflammatory cells, alongwith vacuolar degenerative changes of renal tubule cells on ACR exposure. In addition, Hashimoto and Sakamoto in 1982, reported degenerative changes in reticulocytes and bone marrow cells in mice and rats, which could also affect renal tubules.18 Thus, acrylamide, being a highly toxic substance with a high potential of causing damage to internal organs, this study was conducted to explore the effect of acrylamide on renal tissue in a subacute level of exposure and study the comparative protective effect of Vit-E and 5-ASA treatment on ACR toxicity so as to develop them as potential ameliorative agents.
Methods
Materials
Plus one ACR (PAGE) grade with purity >99.95% was purchased from Pharmacia Biotech (Upsala, Swedan). The 5-ASA 95% was purchased from Sigma-Aldrich (Steinheim, Germany). Vitamin-E (DL-a-tochopherol Acetate), >98% (HPLC) was purchased from Sigma-Aldrich (Steinheim, Germany). Unless otherwise mentioned, all other chemicals and materials were purchased from BHD laboratory supplies (Analar, England) and were of molecular biology grade.
Animals and treatments
A total of 49 adult male Wister rats 250 ± 20 gram (g), aged 60 days, were purchased from King Fahd Medical Research Center in Jeddah, Kingdom of Saudi Arabia (KSA), and were housed as 4 per polycarbonate cage with wood shaving as bedding. Animals were kept under a controlled environment at 22 ± 2°C and relative humidity at 40-65% and 12 hours/12 hours light dark cycles throughout the experimental period. The rats were fed with normal laboratory chow. Tap water in plastic bottles with steel sipper tubes were used for an ad libitum supply of water. The use of animals and experimental design were approved by unit of biomedical ethics, King Abdulaziz University Medical College, Jeddah, KSA, which are in compliance with the national and international laws and policies (7th edition). All procedures were performed according to the National Institutes of Health Guiding Principles in the Care and Use of Animals. Animals were allowed to acclimatize at the experimental environment for 3 days before dosing initiation. The animals were randomly divided into 7 groups (n=7 each). One control group the 3 ACR treated groups (ACR alone, ACR+ 5-ASA, ACR + Vit-E, and ACR +5-ASA +Vit-E) and 2 groups for Vit-E alone and 5-ASA alone. The dose of ACR used was 45mg/kg bw/day for 5 consecutive days, which was selected after exhaustive review of literature for minimal dose of ACR causing adverse effects without producing major neurotoxicity.10 Dosing solutions were daily prepared using distilled water. The ACR treated rats were dosed with ACR by oral gavage using curved-ball ended metallic needle (Size PS-18). Control group was gavaged with 1 ml of distilled water. vit-E was administered at a dose of 200mg/kg bw/day according to by oral gavage. This was carried out in accordance to Takhshid et al.19 followed by Takhshid et al,19 5-ASA was injected intra peritoneally at 25 mg/kg bw/day for 5 consecutive days. All animals were weighed and observed for mortality or any behavioural changes once per day during the dosing and recovery period. After 5 days of treatment, 2 ml of blood was collected from retro-orbital sinus in plain tubes. Blood samples were centrifuged at 3200 g for 10 minutes. A recovery period of 1 day after ACR cessation was given before the animals were killed by cervical dislocation. Right kidney was isolated for further experimental evaluation.
Methodology
A comprehensive review of literature to find prior related research was carried out by searching Science Direct and PubMed, and all the related information was gathered and recorded. The present study was carried out between August and November 2015 at King Fahad Medical Research Centre, King Abdulaziz University, Jeddah, KSA. Inclusion criteria considered in this study for each rat were as body weight of 250 g, age of 60 days and in good health. Exclusion criteria of each rat were as body weight less than 230 g, aeging less than 60 days and showing signs of disease.
Biochemical analysis, lactate dehydrogenase (LDH) assay
Lactate dehydrogenase was evaluated by using quantitative data measurements obtained by Dimension Vista® System and Flex® reagent cartridge. Siemens Healthcare Diagnostics, Inc, Newark DE The reaction took place in a 96 micro-well plate where all reagents are ready to use liquid solutions and the available wells that numbered from one to 8 contained different concentration of N-Methyl-D-glucamine, L(+)-Lactate and NaCl while the last 4 wells involved with β-Nicotinamide Adenine Dinucleotide (NAD) and Nicotinamide Adenine Dinucleotide Lithium Salt, added with preservatives and stabilizers. The enzyme linked immunoassay began with alkaline buffer solution of pH 9.4 that included a substrate. The presence of NAD+ helps LDH to oxidize the substrate, which results in the formation of pyruvate and NADH that absorbs light at 340 nm and can be measured. These readings can be measured at 340/700 nm and they are directly proportionate to the lactate dehydrogenase levels in the serum.

Statistical analysis
All statistical analysis were performed using The Statistical Package for the Social Sciences (SPSS Inc., Chicago, IL, USA) version 16. Data were expressed as mean ± 2SD, where SD stands for standard deviation (SD). Differences among the groups were analyzed by one-way analysis of variance (ANOVA) followed by the Tukey’s test as a post hoc for multiple comparisons. A p-value of less than 0.05 was considered as criterion for a statistically significant difference. In our results, we got a p-value of 0.07 for all experiments involving body weight changes and 0.08 for experiments involving LDH assay.
Results
Pseudom general observation
Rats treated with a dose of 45 mg/kg of ACR showed a rough coat and signs of aggression, with reduction in food and water intake. Improvement in water and food intake was detected in the group treated with ACR and 5-ASA. Rats in the control group showed no symptoms of illness or mortality during the experimental period. Also, no mortality was recorded among ACR treated or other group.
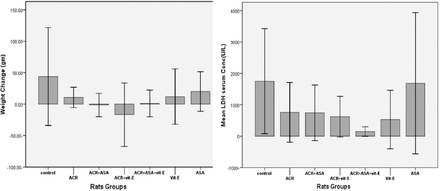
Effect of ACR and antioxidants on rats body weight changes and lactate dehydrogenase activity
No significant changes were observed as summarized in Figure 1.
Effect of acrylamide (ACR) and antioxidants 5-aminosalicylic acid (ASA), vitamin E (vit-E), on body weight changes and lactate dehydrogenase activity, LDH - lactate dehydrogenase.
Histopathology of the kidney
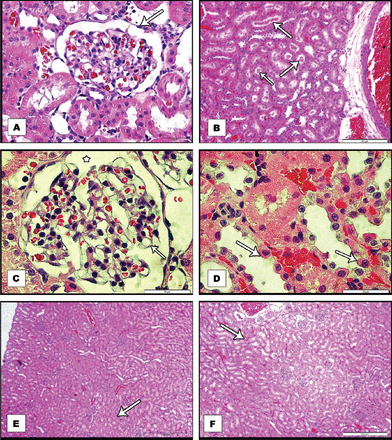
The kidney was taken from control rat shows the cortex as alternating areas of convoluted tubules and renal corpuscles, which constitute glomerular capsules (Bowman’s capsule) and glomeruli Numerous tubules sectioned in various planes lying near to renal corpuscles can be identified. The proximal convoluted tubules with relatively small lumen and broad cuboidal cells having brush borders are seen (Figure 2A). Distal convoluted tubules are fewer in number having large regular lumen with smaller cuboidal cells. However, in the group treated with 45 mg/kg bw of ACR for 5 sequential days, acute lesion in the kidney cortex was observed, particularly in the proximal convoluted tubules. Acute tubular necrosis was seen in the distal tubules, which were obstructed by casts formed by tubular debris. Many distal tubules were also seen distended with proteinaceous casts (Figure 2B).
Sections of kidneys isolated from: A) control adult rat, with normal Bowman’s space. B& C& E) acrylamide treated rat, B) Shows protein casts (arrows) in the lumen of the tubule with tubular degeneration C) Shows segmental duplication of the glomerular basement membrane together with endothelial swelling D) High power view, shows extensive tubular damage, with, cytoplasmic hyperoesinophilia, enlarged hyperchromatic nuclei (arrows) and nucleoli E) acrylamide +5-aminosalycylic acid treated rat, Shows good protection of the kidney with normal renal corpuscles and minimal congestion and F) acrylamide + 5-aminosalicylic acid + vitamin-E treated rat. Shows protection with minimal tubular dilatation. All sections were stained with H&E stain and viewed with light microscopy.
Further, segmental duplication of the glomerular basement membrane together with endothelial swelling, shrunken glomerular capillary tufts and distended Bowman’s capsule was noted in the kidneys of ACR treated group (Figure 2C). A high dose of ACR also caused extensive proximal tubular necrosis characterized by partial loss of epithelial lining cells in many tubules, including epithelial simplification, loss of brush, cytoplasmic hyperoesinophilia, enlarged hyper chromatic nuclei and nucleoli (Figure 2D) In the group treated with ACR and 5-ASA, good protection was noted in the form of normal corpuscles, however minimal congestion was still observed (Figure 2E). In the group treated with ACR and vitamin-E the protection was not as good as with 5-ASA, while vitamin-E supplementation alone also did not show effective protection against ACR induced nephrotoxicity. Interestingly, the protection of the kidney was maximum in the group involving treatment with both antioxidants (5-ASA+vitamin-E) as normal histological structure of the kidney was observed (Figure 2F). Treatment of the rats with 5-ASA and vitamin-E alone over this short period of experiment does not show any major defect in the normal histology of the kidney (not shown).
Discussion
Acrylamide was investigated as a potential renal toxin and the comparative protective effects of Vit-E and 5-ASA on ACR induced changes in rats kidney were explored. The ACR is known to induce structural and functional alterations in many organs, however, only a few toxicity studies of ACR on renal system are available.
The effects of ACR and antioxidants on rats body weight changes
This study showed that concomitant administration of ACR (daily) for 5 days at dose of 45 mg/kgbw/day, has no significant effect on body weight change. Our finding is in line with the that of Lafferty J et al20 showing similar result. However, some other studies conducted on ACR alone revealed a significant decrease in body weight gain and food intake in experimental animals after administrated of ACR.11 This could be due to short duration of ACR exposure in our study.
The effect on LDH
lactate dehydrogenase, a cellular enzyme, mainly present in the heart, kidney, and liver is released in response to damage of cell membrane caused by any toxic substance.21 Our findings of no significant change in LDH activity coincide with several studies reporting no significant change of LDH after ACR treatment, that investigated small doses of acrylamide (0.5, 5, 25, 50, 250 and 500 µg/kg body weight) in drinking water on rats for 10 weeks.22 This indicates that sub-acute levels of ACR exposure over a short period of time do not cause the release of LDH to detectable limits in serum of the subjects.
Histopathology
The ACR administration, though over short period of time, incapable of causing LDH release showed damaging effect on the histological structure of the kidney in the form of enlarged Bowman’s capsule, accumulation of hyaline droplets and tubular degeneration with intertubular congestion, intertubular congestion in the renal cortex, deposition of protein casts in the lumen of the tubule with tubular degeneration, segmental duplication of the glomerular basement membrane together with endothelial swelling, enlarged Bowman’s space, extensive tubular damage, including epithelial simplification, loss of brush, cytoplasmic hyperoesinophilia, enlarged hyper chromatic nuclei and nucleoli, shrunken glomerular capillary tufts and distended Bowman’s capsule, dilated tubules, and increase in Bowman’s space. Histopathologic changes in the kidney have been reported in other findings as well.9 However, increasing the time of ACR exposure could show more pronounced pathology of the kidney.
Since ACR exposure produces pronounced oxidative stress, an antioxidant supplementation could show amelioration of toxic symptoms induced by ACR.
Both 5-ASA and vitamin E are known to have good anti oxidant properties, 5-ASA being a potent anti-inflammatory agent as well.15 We employed these antioxidants to study their protective role in renal toxicity caused by ACR and also elucidate the comparative effect of therapy. Vitamin E alone did not show significant histological protection in renal toxicity as compared with that shown by 5-ASA. However, a combined administration of the 2 showed dramatic improvement in renal histology of ACR exposed rats. Vitamin E and 5-ASA individually have been shown as efficient ameliorators of ACR toxicity in other tissue systems as well.14,23
In conclusion, our study shows that exposure of rats to sub-acute levels of ACR causes histological damage to kidneys, with no significant effect on change in body weight or LDH activity. These symptoms could be reversed to normal by concomitant supplementation of 5-ASA. A combination of vitamin-E and 5-ASA supplementation, however, showed enhanced protective effect in ACR induced renal toxicity. This study gives an insight on the toxic effects of ACR in sub-acute levels to kideny and marks a combination of vitamin-E and 5-ASA to be protective against ACR- toxicity. However, this study did not make an in-depth analysis of damage caused by ACR in kidneys since the time period of ACR exposure was short and no dose-dependent outcomes were studied.
Acrylamide produced histopathologic changes in sub-acute levels emphasizes it to be highly toxic to renal system. An important finding of this study was that 5-ASA could be developed as a potential curative agent for ACR induced renal toxicity, either in combination with vitamin-E or by selection of an effective dose of itself. Further investigations over long period of time with concentration variations of ACR are required to set a limit of exposure marking it as a renal toxin. A dose-dependent study on supplementation of 5-ASA and vitamin-E together could be utilized in drug development.
Footnotes
Disclosure. Authors have no conflict of interest, and the work was not supported or funded by any drug company.
- Received August 8, 2016.
- Accepted November 9, 2016.
- Copyright: © Saudi Medical Journal
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.