Abstract
Micro-ribonucleic acids (miRNAs) are small (21-25 nucleotide) single-stranded, evolutionarily conserved non-protein-coding RNAs, which control diverse cellular functions by interacting with the 3’ untranslated region of specific target messenger RNAs at the post-transcriptional level. Research shows that an aberrant expression profile of miRNAs has been linked to a series of diseases, including hypertension. In the past few decades, it has been demonstrated that excessive activation of the renin-angiotensin aldosterone system (RAAS) involves in the pathogenesis of hypertension. This article reviews the latest insights in the identification of RAAS-correlative miRNAs and the potential mechanisms for their roles in hypertension.
Hypertension is one of the most common diseases in the world. If not detected in time and treated appropriately, sustained high blood pressure will cause serious heart, brain, kidney, and other organ damage or diseases, such as myocardial infarction, heart failure, renal failure, and stroke.1 Studies have shown that in adults, the average prevalence of hypertension is approximately 30-45% in Europe, and rises sharply with increase in age.2 According to the predicted increase in global hypertension prevalence of approximately 10%, during the years 2000-2025, an estimated 560 million extra people will be affected by the disease.3
Hypertension is a complex and multifactorial disease. Several mechanisms have been implicated in its pathogenesis. These include: overactivity of the renin-angiotensin aldosterone system (RAAS) and sympathetic nervous system, hypertrophy, endothelial dysfunction, oxidative stress and others.4 Micro-ribonucleic acids (miRNAs) are small (21-25 nucleotide) single-stranded, evolutionarily conserved non-protein-coding RNAs, several of which participate in the pathogenesis of hypertension.4 Recent research shows that compared with their healthy counterparts, approximately 27 miRNAs were differentially expressed in hypertensive patients.5 The purpose of the present review is to focus on recent data regarding miRNAs that directly target multiple components of RASS in hypertension (Table 1).
Renin-angiotensin aldosterone system related microRNAs in hypertension according to a study from China.
MiRNAs source and function
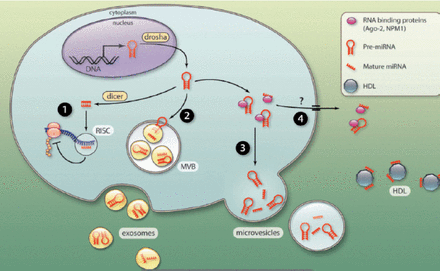
Since their discovery by Lee et al6 in 1993, miRNAs have become one of the leading research areas of the past 2 decades. MicroRNAs are produced naturally by eukaryotes cells.7 MiRNAs come from individual miRNA genes or introns of protein-coding genes. They are initially transcribed by RNA-polymerase II to yield long primary transcripts (pri-miRNAs). Subsequently, Most are spliced by the Drosha RNase III-cofactors complex in the nucleus.8-11 The pri-miRNAs are transported out of the nucleus by exportin-5,12 and cleaved by Dicer RNA-processing ribonuclease III to form a double stranded RNA molecule (miRNA-miRNA* duplex). Only one strand is preferentially retained in the complex and becomes the mature miRNA (Figure 1). The other is degraded and eliminated from the miRNA-miRNA* duplex.9,10,13
The mature miRNA (single-stranded) is loaded into the RNA-induced silencing complex, where it can promote mRNA silencing by degradation of mRNA, or by blocking protein translation. The major effect involves targeting of the miRNA to the 3’ untranslated region (UTR) of mRNA transcripts.9,10,14 During the past 2 decades, miRNAs have been extensively investigated, and it is now recognized that miRNAs play a significant role in regulating fundamental cellular processes, including cell proliferation, differentiation, apoptosis, migration, as well as other effects.7,9
Renin-angiotensin aldosterone system and hypertension
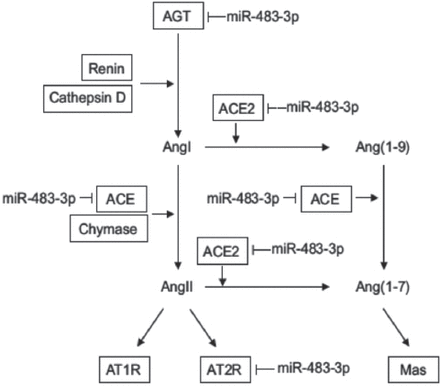
The RAAS regulates a variety of physiological functions, such as hemodynamic equilibrium, electrolyte balance, and circulating volume.15 Nevertheless, overactivation of the RAAS is central to the pathogenesis of hypertension. As a hormone system, RAAS contains several enzymes, peptides and receptors.16 The RAAS cascade begins when renal juxtaglomerular (JG) cells secrete renin into the circulation (Figure 2).16,17 Plasma renin (produced principally by the kidney) cleaves the peptide angiotensin I (Ang I) from angiotensinogen (produced principally in the liver). Subsequently, Ang I is cleaved by angiotensin converting enzyme (ACE) to an 8 amino acid peptide, angiotensin II (Ang II).16,17
The microRNAS that target components of the renin-angiotensin aldosterone system. AGT - Ang II type, AGT1R - Ang II type 1 receptor, AGT2R - Ang II type 2 receptor, Ang I - angiotensin I, Ang II - angiotensin 2, MR - mineralocorticoid receptor. Reproduced from: Obama T, Eguchi S. MicroRNA as a novel component of the tissue renin angiotensin system. J Mol Cell Cardiol 2014; 75: 98-99.48 With permission from Elsevier.
Ang II is the principal biological effector and plays a significant role in raising blood pressure by its own independent effects of sodium reabsorption in the kidney, as well as acts directly on the arterioles to cause vasoconstriction, acts on brain to stimulate thirst, acts on sympathetic nerve endings to increase sympathetic tone. Ang II can also act on the adrenal cortex to promote the production of aldosterone, which increases reabsorption of sodium and chloride in the renal proximal tubules.17-19 However, it is renin that mediates the rate-limiting step in the production of Ang II. Renin release is regulated by an intrarenal baroreceptor that detects pressure in the afferent arteriole, by β-adrenergic sympathetic innervation and the delivery of salt (especially Cl) in the tubular fluid.17,18,20
MicroRNAs that decrease blood pressure
MicroRNA-181a (miR-181a)
Overactivity of the sympathetic nervous system may be involved in the pathogenesis of hypertension. Increased renal sympathetic nerve activity can also stimulate the secretion of renin, which as mentioned above, mediates the rate-limiting step in the production of Ang II and therefore, raise blood pressure.21 Hypertension in genetically hypertensive (BPH/2J) mice involves overactivity of the sympathetic nervous system. Jackson et al22 found that there was a negative correlation between miR-181a and renin in BPH/2J mice. Marques et al23 concluded that in kidneys, if hypertensive patients expression of miR-181a was decreased and this was accompanied by an increase in renin mRNA, this will lead to an increase in blood pressure. Normally, expression of miR-181a, by destabilizing renin mRNA, keeps renin low. Therefore, increasing the expression of miR-181a may decrease blood pressure by suppressing the renal sympathetic nerve activity and decreasing the secretion of renin.
MicroRNA-145 (miR-145)
The Ang II, effector of the RAAS, is produced when ACE hydolyzes Ang I. Increased ACE expression is associated with high blood pressure. Interestingly, the expression of ACE is down-regulated by shear stress. Kohlstedt et al24 found that the shear stress-mediated ACE expression is down-regulated by miR-145(located on chromosome 5q33).24,25 Hu et al26 have found that overexpression of miR-145 induced down-regulation of ACE protein but that this was not via a reduction in ACE mRNA level. Overexpression of miR-145 contributes to up-regulation of ACE, as well as increasing blood pressure, by alternate post-transcriptional effects.25,26 Activating the ERK1/2 signaling pathway can suppress the expression of miR-145, promote ACE expression, and increase blood pressure.26
MicroRNA-132 (miR-132) and microRNA-212 (miR-212)
MicroR-132 and miR-212 are closely located together on chromosome 17p13 and co-regulate their common promoters and regulatory sequences.27,28 Eskildsen et al28 found that after 10 days of sustained Ang II-induced hypertension in rats, the expression of miR-132/-212 increased in myocardium, arteries, and kidney. There is a positive correlation between the degree of increase in miR-132/miRNA-212 and blood pressure both in vitro and in vivo. Furthermore, they demonstrated substantial decreases in miR-132 and miR-212 expressions by inhibition of the Gαq subunit in cardiac fibroblasts. This demonstrated that miR-132 and miR-212, via activation of the Gαq-dependent signaling pathway (Gαq is the functional subunit of Gq protein, a member of the G protein family), takes part in Ang II-induced hypertension.28 This study provided a novel perspective in hypertensive disease mechanisms.
MicroRNA-155 (miR-155)
Angiotensin II has 2 types receptors: Ang II type 1 receptor (AGT1R) and Ang II type 2 receptor (AGT2R). The AGT1R takes part in a variety of physiological and pathological mechanisms involved with the cardiovascular control. These include vasoconstriction, the release of catecholamines, and elevation of blood pressure. In contrast AGT2R is part of the “protective arm of the RAAS” and decreases blood pressure.29 The gene for AGT1R contains 5 exons and is located on chromosome 3q.30 Recent studies have shown that miR-155 (located on chromosome 21) can regulate the expression of AGT1R mRNA by targeting the 3’-untranslated region to silence AGT1R mRNA expression.31 In lung fibroblasts, miR-155 inhibits the expression level of AGT1R and the effect can be repressed by transforming growth factor-β1 (TGF-β1) or anti-miR-155 leading to an increase in AGT1R protein levels.32 In addition, an increase in expression of miR-155 decreased AGT1R protein and was associated with lower blood pressure in trisomy 21 patients.33 This demonstrated that the expressions level of miR-155 negatively correlate with AGT1R protein levels and blood pressure.
MicroRNA-124 (miR-124) and microRNA-135a (miR-135a)
The mineralocorticoid receptor (MR) gene (NR3C2) is located on chromosome 4q31.23 and is involved in hypertension by promoting renal salt retention and influencing salt appetite.34,35 The NR3C2 deficient mice died in the neonatal period because of severe sodium and water loss.36 Recent studies have demonstrated that miR-124 and miR-135a could suppress the expression of NR3C2 by reducing the amount of mineralocorticoid receptor protein rather than at the mRNA level.34,36 Therefore, miR-124 and miR-135a participate in reduction of blood pressure via modulation of the functioning of RAAS.
MicroRNAs which increase blood pressure
MicroRNA-421 (miR-421) and microRNA-143 (miR-143)
Since the discovery by Donoghue et al37 in 2000, angiotensin converting enzyme 2 (ACE2) has emerged as a negative regulator of the RAAS and participates in the pathophysiology of hypertension. Ang II is directly cleaved by ACE2 to give angiotensin (1-7), whose function is opposite to ACE/Ang II /AGT1R signaling.38,39 Lambert et al40 confirmed that miR-421 (located on chromosome X q13.2) can decrease the expression of the ACE2 protein but with no change of the ACE2 homologue ACE. The miR-421 had no effect on ACE2 mRNA levels, which suggest that miR-421 decreases the expression of the ACE2 protein and down-regulates the RAAS (thereby decreases blood pressure) by translational repression.40-42 Gu et al43 found that chronic aerobic exercise training improved RAAS balance and decreased blood pressure in spontaneously hypertensive rats by down-regulation of the expression of miR-143, which was accompanied by significantly elevated circulating ACE2 and angiotensin (1-7) levels.
MicroRNA-483-3p (miR-483-3p)
Kemp et al44 found that miR-483-3p (located on chromosome 11) is a novel Ang II-regulated miRNA that inhibited the expression of luciferase reporters bearing 3’-UTRs of 4 different RAAS-related genes (angiotensinogen, ACE-1, ACE-2 and AGTR2), which was reversed by antago-mir-483-3.44,45 Suppressing angiotensinogen and ACE-1 would eventually block production of Ang II and decrease blood pressure. Nevertheless, evidence shows that AGT2R and ACE-2 oppose the AGT1R-mediated vasoconstrictor action of Ang II, being a part of the “protective arm of RASS”.46 Therefore, suppressing AGT2R and ACE-2 would eventually increase blood pressure. It suggests that miR-483-3p will ultimately increase, or decrease blood pressure by directly targeting multiple components of the RAAS. More studies are needed to demonstrate, which has the stronger role, and thus whether the net effect will be an increase or a decrease in blood pressure.
In conclusion, the discovery of the interaction between RAAS components and novel miRNAs, and their role in blood pressure control and hypertension, represents a major milestone in hypertensive research. However, the functional roles of the RAAS-related miRNAs will require further investigations to elucidate the interactions and precise mechanisms of the involvement of miRNAs and RAAS in hypertension. We predict that RAAS-related miRNAs will be used as diagnostic biomarkers and therapeutic targets for hypertensive patients in the future.
Footnotes
Disclosure. Authors have no conflict of interests, and the work was not supported or funded by any drug company. This work was supported by the National Natural Science Foundation of China (Grant No. 81170133 [to J. Yang]; Grant No. 81200088,81470387 [to J. Yang]), and the Natural Science Foundation of Yichang city, China (Grant No. A12301-01), as well as Hubei Province’s Outstanding Medical Academic Leader Program, Hubei, China.
- Copyright: © Saudi Medical Journal
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.